
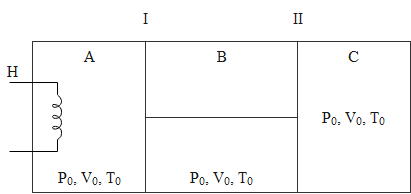
The figure shows an insulated cylinder divided into three parts $A$, $B$ and $C$. Piston I and II are connected by a rigid rod and can move without friction inside the cylinder. Piston I is perfectly conducting while piston II is perfectly insulating. The initial state of gas $(\gamma=1.5)$ present in each compartment A, B and $C$ is as shown. Now, compartment A is slowly given heat through a heater $\mathrm{H}$ such that the final volume of $\mathrm{C}$ becomes $\dfrac{4 V_{0}}{9} .$ Assume the gas to be ideal and find the heat supplied by the heater.
(A) $18 P_{o} V_{o}$
(B) $12 P_{o} V_{o}$
(C) $9 P_{o} V_{o}$
(D) $25 P_{o} V_{o}$
Answer
233.1k+ views
HintFirst we need to apply the heat supply equation to determine the heat change in case of each compartment. Then equating the temperature and pressure, we will get the heat supplied by the heater.
Complete step by step answer:
We can know the heat supplied from the equation,
$\Rightarrow \Delta \mathrm{Q}=\Delta \mathrm{U}+\Delta \mathrm{W}$
$\Rightarrow \Delta \mathrm{U}=\Delta \mathrm{U}_{\mathrm{A}}+\Delta \mathrm{U}_{\mathrm{B}}+0 \quad$
We find that there is no heat change in case of compartment C
$\Rightarrow \Delta \mathrm{W}=\Delta \mathrm{W}_{\mathrm{A}}+0+\Delta \mathrm{W}_{\mathrm{C}} \quad$
We find that there is no change in volume in case of compartment B hence work done = 0
The initial conditions are assumed as
$\Rightarrow \mathrm{P}_{\mathrm{o}}$ for Pressure
$\Rightarrow \mathrm{V}_{\mathrm{o}}$for Volume
$\Rightarrow \mathrm{T}_{\mathrm{o}}$for Temperature
The final conditions in respective compartments:
In Compartment C
$\Rightarrow \mathrm{PV}^{\gamma}=\mathrm{PV}^{\gamma}=\mathrm{P} \times\left(\dfrac{4 \mathrm{V}_{0}}{9}\right)^{1.5}=\mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}^{1.5}$
$\Rightarrow P=\dfrac{27 P_{0}}{8}$
$\Rightarrow \dfrac{\mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}}{\mathrm{T}_{\mathrm{o}}}=\dfrac{\dfrac{27 \mathrm{P}_{\mathrm{o}}}{8} \times \dfrac{4 \mathrm{V}_{\mathrm{o}}}{9}}{\mathrm{T}}$
$\Rightarrow \mathrm{T}=\dfrac{3 \mathrm{T}_{\mathrm{o}}}{2}$
In Compartment A
$\Rightarrow \mathrm{P}=\dfrac{27 \mathrm{P}_{\mathrm{o}}}{8}$
For pistons to come at rest both compartment A and C have to have the same pressure.
$\Rightarrow \dfrac{\mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}}{\mathrm{T}_{\mathrm{o}}}=\dfrac{\dfrac{27 \mathrm{P}_{\mathrm{o}}}{8} \times\left(\mathrm{V}_{\mathrm{o}}+\dfrac{5 \mathrm{V}_{\mathrm{o}}}{9}\right)}{\mathrm{T}}$
$\Rightarrow \mathrm{T}=\dfrac{21 \mathrm{T}_{\mathrm{o}}}{4}$
In Compartment B
$\Rightarrow \mathrm{T}=\dfrac{21 \mathrm{T}_{\mathrm{o}}}{4}$
We know that the temperature of both compartment A and compartment B should be the same at equilibrium. Therefore,
$\Rightarrow \dfrac{\mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}}{\mathrm{T}_{\mathrm{o}}}=\dfrac{\mathrm{P} \times \mathrm{V}_{\mathrm{o}}}{\dfrac{21 \mathrm{T}_{\mathrm{o}}}{4}}$
$\Rightarrow P=\dfrac{21 P_{0}}{4}$
$\Rightarrow \gamma=\dfrac{\mathrm{C}_{\mathrm{p}}}{\mathrm{C}_{\mathrm{v}}}=\dfrac{\mathrm{f}+2}{\mathrm{f}}$
$\Rightarrow \mathrm{f}=4$
$\Rightarrow \mathrm{C}_{\mathrm{v}}=\dfrac{\mathrm{fR}}{2}=2 \mathrm{R}$
$\Rightarrow \Delta \mathrm{U}_{\mathrm{A}}=\Delta \mathrm{U}_{\mathrm{B}}$
$\Rightarrow \Delta \mathrm{U}_{\mathrm{A}}=\mathrm{n} \mathrm{C}_{\mathrm{v}} \Delta \mathrm{T}$
$\Rightarrow \Delta \mathrm{U}_{\mathrm{A}}=\dfrac{\mathrm{P}_{0} \mathrm{V}_{\mathrm{o}}}{\mathrm{RT}_{\mathrm{o}}} \times \mathrm{C}_{\mathrm{v}}\left(\dfrac{21 \mathrm{T}_{\mathrm{o}}}{4}-\mathrm{T}_{\mathrm{o}}\right)=\dfrac{\mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}}{\mathrm{RT}_{\mathrm{o}}} \times 2 \mathrm{R} \times \dfrac{17 \mathrm{T}_{\mathrm{o}}}{4}=\dfrac{17 \mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}}{2}$
$\Rightarrow \Delta \mathrm{W}_{\mathrm{A}}=-\Delta \mathrm{W}_{\mathrm{C}}$
as the gas in chamber A is working on chamber C
$\Rightarrow \Delta \mathrm{Q}_{\mathrm{C}}=0$ as it is an adiabatic process
Therefore,
$\Rightarrow \Delta \mathrm{Q}_{\mathrm{C}}=\Delta \mathrm{U}_{\mathrm{C}}+\Delta \mathrm{W}_{\mathrm{C}}$
$\Rightarrow \Delta \mathrm{U}_{\mathrm{C}}=-\Delta \mathrm{W}_{\mathrm{C}}=\mathrm{n} \mathrm{C}_{\mathrm{v}} \Delta \mathrm{T}=\dfrac{\mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}}{\mathrm{RT}_{\mathrm{o}}} \times 2 \mathrm{R} \times\left(\dfrac{3 \mathrm{T}_{\mathrm{o}}}{2}-\mathrm{T}_{\mathrm{o}}\right)=\mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}$
$\Rightarrow \Delta \mathrm{U}=\Delta \mathrm{U}_{\mathrm{A}}+\Delta \mathrm{U}_{\mathrm{B}}+0 \quad$
Therefore, no heat change occurs in case of compartment C
$\Rightarrow \Delta \mathrm{W}=\Delta \mathrm{W}_{\mathrm{A}}+0+\Delta \mathrm{W}_{\mathrm{C}} \quad$
Hence, no change in volume in case of compartment B and therefore work done =0
The heat supplied by the heater = The heat supplied to compartment A + The heat flow through piston
$\Rightarrow \Delta \mathrm{Q}=\Delta \mathrm{U}_{\mathrm{A}}+\Delta \mathrm{U}_{\mathrm{B}}+\Delta \mathrm{W}_{\mathrm{AB}}=\Delta \mathrm{U}_{\mathrm{A}}+\Delta \mathrm{U}_{\mathrm{B}}+\Delta \mathrm{W}_{\mathrm{A}}=2 \Delta \mathrm{U}_{\mathrm{A}}+\Delta \mathrm{W}_{\mathrm{A}}$
$\Rightarrow \Delta \mathrm{Q}=18 \mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}$
Therefore, the correct answer is Option A.
Note: We must have a thorough knowledge on thermodynamics and we must read the given figure carefully. At first glance, the image might seem to be like a circuit but we must read the question thoroughly before deducing.
Complete step by step answer:
We can know the heat supplied from the equation,
$\Rightarrow \Delta \mathrm{Q}=\Delta \mathrm{U}+\Delta \mathrm{W}$
$\Rightarrow \Delta \mathrm{U}=\Delta \mathrm{U}_{\mathrm{A}}+\Delta \mathrm{U}_{\mathrm{B}}+0 \quad$
We find that there is no heat change in case of compartment C
$\Rightarrow \Delta \mathrm{W}=\Delta \mathrm{W}_{\mathrm{A}}+0+\Delta \mathrm{W}_{\mathrm{C}} \quad$
We find that there is no change in volume in case of compartment B hence work done = 0
The initial conditions are assumed as
$\Rightarrow \mathrm{P}_{\mathrm{o}}$ for Pressure
$\Rightarrow \mathrm{V}_{\mathrm{o}}$for Volume
$\Rightarrow \mathrm{T}_{\mathrm{o}}$for Temperature
The final conditions in respective compartments:
In Compartment C
$\Rightarrow \mathrm{PV}^{\gamma}=\mathrm{PV}^{\gamma}=\mathrm{P} \times\left(\dfrac{4 \mathrm{V}_{0}}{9}\right)^{1.5}=\mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}^{1.5}$
$\Rightarrow P=\dfrac{27 P_{0}}{8}$
$\Rightarrow \dfrac{\mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}}{\mathrm{T}_{\mathrm{o}}}=\dfrac{\dfrac{27 \mathrm{P}_{\mathrm{o}}}{8} \times \dfrac{4 \mathrm{V}_{\mathrm{o}}}{9}}{\mathrm{T}}$
$\Rightarrow \mathrm{T}=\dfrac{3 \mathrm{T}_{\mathrm{o}}}{2}$
In Compartment A
$\Rightarrow \mathrm{P}=\dfrac{27 \mathrm{P}_{\mathrm{o}}}{8}$
For pistons to come at rest both compartment A and C have to have the same pressure.
$\Rightarrow \dfrac{\mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}}{\mathrm{T}_{\mathrm{o}}}=\dfrac{\dfrac{27 \mathrm{P}_{\mathrm{o}}}{8} \times\left(\mathrm{V}_{\mathrm{o}}+\dfrac{5 \mathrm{V}_{\mathrm{o}}}{9}\right)}{\mathrm{T}}$
$\Rightarrow \mathrm{T}=\dfrac{21 \mathrm{T}_{\mathrm{o}}}{4}$
In Compartment B
$\Rightarrow \mathrm{T}=\dfrac{21 \mathrm{T}_{\mathrm{o}}}{4}$
We know that the temperature of both compartment A and compartment B should be the same at equilibrium. Therefore,
$\Rightarrow \dfrac{\mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}}{\mathrm{T}_{\mathrm{o}}}=\dfrac{\mathrm{P} \times \mathrm{V}_{\mathrm{o}}}{\dfrac{21 \mathrm{T}_{\mathrm{o}}}{4}}$
$\Rightarrow P=\dfrac{21 P_{0}}{4}$
$\Rightarrow \gamma=\dfrac{\mathrm{C}_{\mathrm{p}}}{\mathrm{C}_{\mathrm{v}}}=\dfrac{\mathrm{f}+2}{\mathrm{f}}$
$\Rightarrow \mathrm{f}=4$
$\Rightarrow \mathrm{C}_{\mathrm{v}}=\dfrac{\mathrm{fR}}{2}=2 \mathrm{R}$
$\Rightarrow \Delta \mathrm{U}_{\mathrm{A}}=\Delta \mathrm{U}_{\mathrm{B}}$
$\Rightarrow \Delta \mathrm{U}_{\mathrm{A}}=\mathrm{n} \mathrm{C}_{\mathrm{v}} \Delta \mathrm{T}$
$\Rightarrow \Delta \mathrm{U}_{\mathrm{A}}=\dfrac{\mathrm{P}_{0} \mathrm{V}_{\mathrm{o}}}{\mathrm{RT}_{\mathrm{o}}} \times \mathrm{C}_{\mathrm{v}}\left(\dfrac{21 \mathrm{T}_{\mathrm{o}}}{4}-\mathrm{T}_{\mathrm{o}}\right)=\dfrac{\mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}}{\mathrm{RT}_{\mathrm{o}}} \times 2 \mathrm{R} \times \dfrac{17 \mathrm{T}_{\mathrm{o}}}{4}=\dfrac{17 \mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}}{2}$
$\Rightarrow \Delta \mathrm{W}_{\mathrm{A}}=-\Delta \mathrm{W}_{\mathrm{C}}$
as the gas in chamber A is working on chamber C
$\Rightarrow \Delta \mathrm{Q}_{\mathrm{C}}=0$ as it is an adiabatic process
Therefore,
$\Rightarrow \Delta \mathrm{Q}_{\mathrm{C}}=\Delta \mathrm{U}_{\mathrm{C}}+\Delta \mathrm{W}_{\mathrm{C}}$
$\Rightarrow \Delta \mathrm{U}_{\mathrm{C}}=-\Delta \mathrm{W}_{\mathrm{C}}=\mathrm{n} \mathrm{C}_{\mathrm{v}} \Delta \mathrm{T}=\dfrac{\mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}}{\mathrm{RT}_{\mathrm{o}}} \times 2 \mathrm{R} \times\left(\dfrac{3 \mathrm{T}_{\mathrm{o}}}{2}-\mathrm{T}_{\mathrm{o}}\right)=\mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}$
$\Rightarrow \Delta \mathrm{U}=\Delta \mathrm{U}_{\mathrm{A}}+\Delta \mathrm{U}_{\mathrm{B}}+0 \quad$
Therefore, no heat change occurs in case of compartment C
$\Rightarrow \Delta \mathrm{W}=\Delta \mathrm{W}_{\mathrm{A}}+0+\Delta \mathrm{W}_{\mathrm{C}} \quad$
Hence, no change in volume in case of compartment B and therefore work done =0
The heat supplied by the heater = The heat supplied to compartment A + The heat flow through piston
$\Rightarrow \Delta \mathrm{Q}=\Delta \mathrm{U}_{\mathrm{A}}+\Delta \mathrm{U}_{\mathrm{B}}+\Delta \mathrm{W}_{\mathrm{AB}}=\Delta \mathrm{U}_{\mathrm{A}}+\Delta \mathrm{U}_{\mathrm{B}}+\Delta \mathrm{W}_{\mathrm{A}}=2 \Delta \mathrm{U}_{\mathrm{A}}+\Delta \mathrm{W}_{\mathrm{A}}$
$\Rightarrow \Delta \mathrm{Q}=18 \mathrm{P}_{\mathrm{o}} \mathrm{V}_{\mathrm{o}}$
Therefore, the correct answer is Option A.
Note: We must have a thorough knowledge on thermodynamics and we must read the given figure carefully. At first glance, the image might seem to be like a circuit but we must read the question thoroughly before deducing.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




