
The electronic configuration of chloride ion is
A) $2\text{ , 8 , 7}$
B) \[2\text{ , }8\text{ , }6\]
C) \[2\text{ , }8\text{ , 8}\]
D) \[2\text{ , }8\text{ }\]
Answer
239.4k+ views
Hint: The electronic configuration can be written in KLMN format. Where the K, L, M, N shells represent the energy shells. The number of electrons accommodated in the shell is represented by the principal quantum number n, where the total number of an electron in the shell is given by the formula $\text{ 2}{{\text{n}}^{\text{2}}}$, where n is the shell number.
Complete step by step solution:
The electronic configuration of an element is a way in which the electrons are distributed in the atomic orbitals. They follow the standard notation in which atomic subshells are placed in increasing order of energy.
The electrons can be distributed in the KLMN based electron shell. The K shell is the first shell or energy level, L is the second shell, M is third, and so on. The KLMN notations indicate the total number of electrons with each principal quantum number which is n.
The total number of electrons accommodated by the energy shell is given by $\text{ 2}{{\text{n}}^{\text{2}}}$, where ‘n’ is the shell number. The values of shell and the principal quantum number is tabulated as:
The neutral chlorine atom has an atomic number of 17. It contains the 17 electrons which are distributed in its atomic shells.
But we are asked to find the electronic configuration of chloride ions.
The chloride ion is obtained when the chlorine atom gains an electron. Now it holds on the one extra electron in its outermost energy shell. Therefore the total number of electrons associated with the chloride is 18. Now we want to accommodate these 18 electrons in energy shells. each shell is subdivided into the subshells.
The chloride ion holds on 18 electrons. These electrons can be distributed in K, L, M, N shells as follows:
The K shell for chloride have one subshell .thus the maximum number of the electron in K shell is, $2{{(1)}^{2}}=\text{ 2}$
Therefore, the K shell or \[\text{ n=1}\]holds two electrons.
The L shell has the 2 subshells which are s and p. Therefore, if we look closely at the electronic configuration of chloride then the total number of electrons in L shells are,
$2{{(2)}^{2}}=\text{ 8}$
Therefore, there are a total of 8 electrons in the L shell as \[\text{ n=2}\]
The M shell has the 3 subshells in it which are s, p, and d. The d-orbital is absent in the chloride atom. Therefore, if we look closely at the electronic configuration of chloride then the total number of electrons in L shells are,
$2{{(3)}^{2}}=\text{ 18}$
But, the chloride ion has an empty d orbital. Therefore the total number of an electron in the M shells is $18-10=8$. Therefore the M shells hold on 8 electrons.
The electron distribution can be depicted as:
\[\text{ }\begin{matrix}
\text{K shell} & \text{=} & \text{2} \\
\text{L shell} & \text{=} & \text{8} \\
\text{M shell} & \text{=} & \text{8} \\
\end{matrix}\text{ }\]
The electronic configuration in terms of K, L, M, and N shells can be represented as:
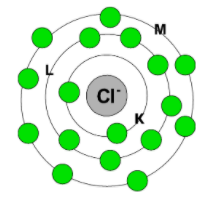
Therefore, the electronic configuration of chloride ion is $2\text{ , 8 , 8}$
Hence, (C) is the correct option.
Note: the electronic configuration of chloride can also be written in form of subshells s, p, d, and f as follows:$\text{1}{{\text{s}}^{\text{2}}}\text{ , 2}{{\text{s}}^{\text{2}}}\text{ , 2}{{\text{p}}^{\text{6}}}\text{ , 3}{{\text{s}}^{\text{2}}}\text{ , 3}{{\text{p}}^{\text{6}}}$ .This configuration is the same as that of the argon but chloride is more reactive compare to the argon.
Complete step by step solution:
The electronic configuration of an element is a way in which the electrons are distributed in the atomic orbitals. They follow the standard notation in which atomic subshells are placed in increasing order of energy.
The electrons can be distributed in the KLMN based electron shell. The K shell is the first shell or energy level, L is the second shell, M is third, and so on. The KLMN notations indicate the total number of electrons with each principal quantum number which is n.
The total number of electrons accommodated by the energy shell is given by $\text{ 2}{{\text{n}}^{\text{2}}}$, where ‘n’ is the shell number. The values of shell and the principal quantum number is tabulated as:
| Shell and ‘n’ value | Max.number of electron |
| K shell, \[\text{ n=1}\] | $2{{(1)}^{2}}=\text{ 2}$ |
| L shell, \[\text{ n=2}\] | $2{{(2)}^{2}}=\text{ 8}$ |
| M shell,\[\text{ n=3}\] | $2{{(3)}^{2}}=\text{ 18}$ |
| N shell,\[\text{ n=4}\] | $2{{(4)}^{2}}=\text{ 32}$ |
The neutral chlorine atom has an atomic number of 17. It contains the 17 electrons which are distributed in its atomic shells.
But we are asked to find the electronic configuration of chloride ions.
The chloride ion is obtained when the chlorine atom gains an electron. Now it holds on the one extra electron in its outermost energy shell. Therefore the total number of electrons associated with the chloride is 18. Now we want to accommodate these 18 electrons in energy shells. each shell is subdivided into the subshells.
The chloride ion holds on 18 electrons. These electrons can be distributed in K, L, M, N shells as follows:
The K shell for chloride have one subshell .thus the maximum number of the electron in K shell is, $2{{(1)}^{2}}=\text{ 2}$
Therefore, the K shell or \[\text{ n=1}\]holds two electrons.
The L shell has the 2 subshells which are s and p. Therefore, if we look closely at the electronic configuration of chloride then the total number of electrons in L shells are,
$2{{(2)}^{2}}=\text{ 8}$
Therefore, there are a total of 8 electrons in the L shell as \[\text{ n=2}\]
The M shell has the 3 subshells in it which are s, p, and d. The d-orbital is absent in the chloride atom. Therefore, if we look closely at the electronic configuration of chloride then the total number of electrons in L shells are,
$2{{(3)}^{2}}=\text{ 18}$
But, the chloride ion has an empty d orbital. Therefore the total number of an electron in the M shells is $18-10=8$. Therefore the M shells hold on 8 electrons.
The electron distribution can be depicted as:
\[\text{ }\begin{matrix}
\text{K shell} & \text{=} & \text{2} \\
\text{L shell} & \text{=} & \text{8} \\
\text{M shell} & \text{=} & \text{8} \\
\end{matrix}\text{ }\]
The electronic configuration in terms of K, L, M, and N shells can be represented as:

Therefore, the electronic configuration of chloride ion is $2\text{ , 8 , 8}$
Hence, (C) is the correct option.
Note: the electronic configuration of chloride can also be written in form of subshells s, p, d, and f as follows:$\text{1}{{\text{s}}^{\text{2}}}\text{ , 2}{{\text{s}}^{\text{2}}}\text{ , 2}{{\text{p}}^{\text{6}}}\text{ , 3}{{\text{s}}^{\text{2}}}\text{ , 3}{{\text{p}}^{\text{6}}}$ .This configuration is the same as that of the argon but chloride is more reactive compare to the argon.
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Difference Between Crystalline and Amorphous Solid: Table & Examples

Types of Solutions in Chemistry: Explained Simply

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Understanding the Angle of Deviation in a Prism

Understanding Electromagnetic Waves and Their Importance

Hybridisation in Chemistry – Concept, Types & Applications

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

CBSE Notes Class 11 Chemistry Chapter 9 - Hydrocarbons - 2025-26

CBSE Notes Class 11 Chemistry Chapter 5 - Thermodynamics - 2025-26

CBSE Notes Class 11 Chemistry Chapter 6 - Equilibrium - 2025-26

CBSE Notes Class 11 Chemistry Chapter 8 - Organic Chemistry Some Basic Principles And Techniques - 2025-26




