
The difference between amylase and amylopectin is __________.
(a)- \[Amylopectin\text{ }have\text{ }1\to 4\alpha -linkage\text{ }and\text{ }1\to 6\beta -linkage\]
(b)- \[Amylopectin\text{ }have\text{ }1\to 4\alpha -linkage\text{ }and\text{ }1\to 6\alpha -linkage\]
(c)- Amylose is made up of glucose and galactose.
(d)- \[Amylose\text{ }have\text{ }1\to 4\alpha -linkage\text{ }and\text{ }1\to 6\beta -linkage\]
Answer
240.6k+ views
Hint: One of the following has the branched-chain structure and one has the linear chain structure. Both of them are made up of glucose components only.
Complete step by step answer:
Starch is not only a single compound but is a mixture of two components-a water-soluble components called amylose (15-20%) and a water-insoluble component called amylopectin (80-85%). The aqueous solution of amylose gives a blue color with an iodine solution. Amylopectin, on the other hand, does not give blue color with iodine solution.
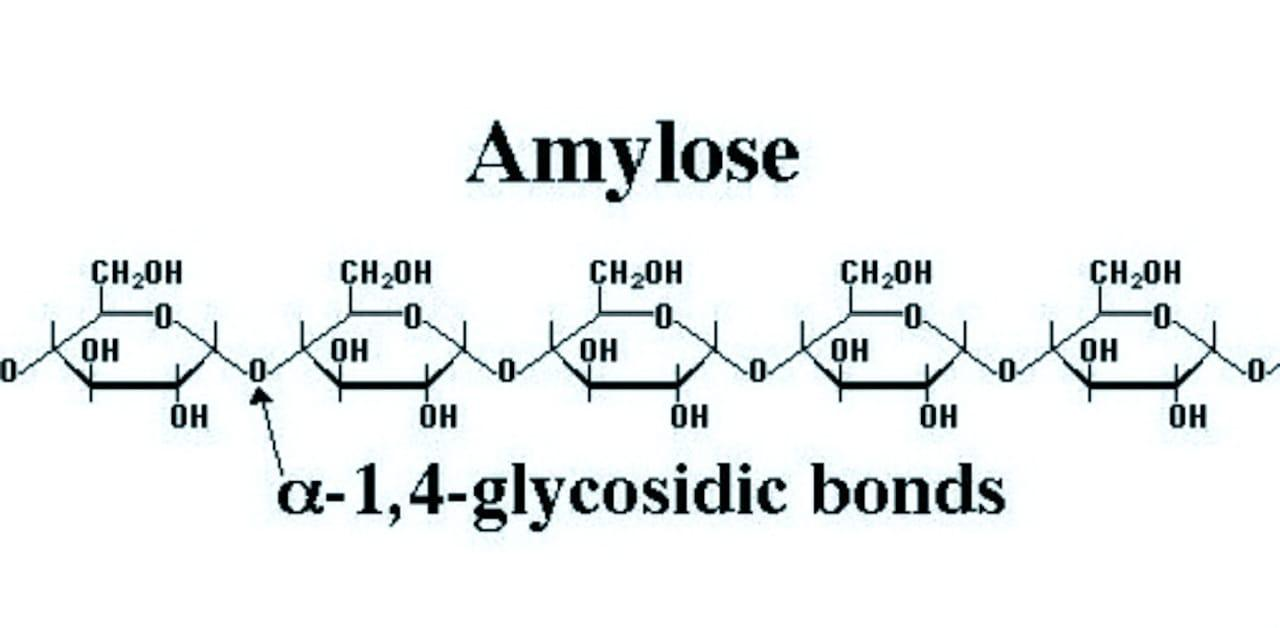
Structure of amylose: It is a linear polymer of \[\alpha -D-glu\cos e\] in which\[{{C}_{1}}\] of one glucose unit is attached to\[{{C}_{4}}\] of the other through\[\alpha -gly\cos idic\] linkage.
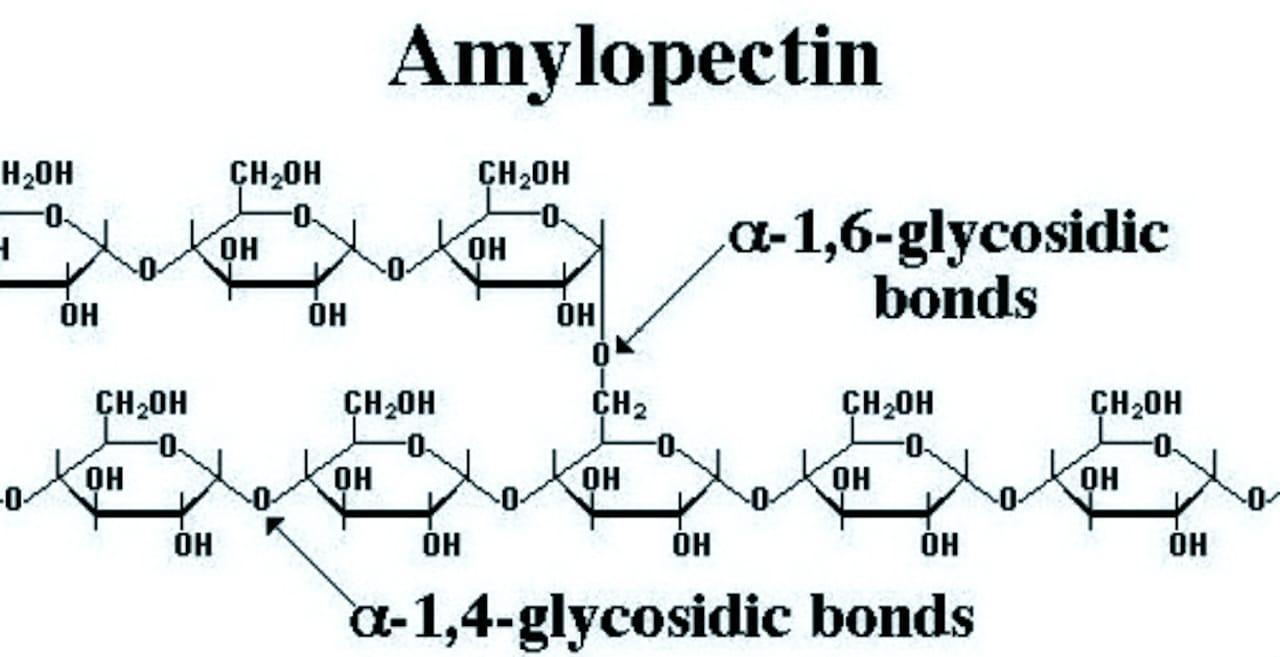
Structure of amylopectin: Amylopectin, on the other hand, is a highly branched polymer. It consists of a large number of short chains each containing 20-25 glucose units which are joined together through \[\alpha -gly\cos idic\] linkage involving \[{{C}_{1}}\] of one glucose unit with\[{{C}_{4}}\] of the other. The \[{{C}_{1}}\]of the terminal glucose unit in each chain is further linked to\[{{C}_{6}}\] of some other glucose unit in the next chain through \[{{C}_{1}}-{{C}_{6}}\]\[\alpha -gly\cos idic\] linkage. This gives amylopectin a highly branched structure.
Hence, from the above discussion option (b) is correct.
Note: You may get confused between \[\alpha -linkage\] and \[\beta -linkage\]. \[\alpha -linkage\] is formed when the bond is formed on the same side and \[\beta -linkage\]is formed when the bond is formed between the opposite side.
Complete step by step answer:
Starch is not only a single compound but is a mixture of two components-a water-soluble components called amylose (15-20%) and a water-insoluble component called amylopectin (80-85%). The aqueous solution of amylose gives a blue color with an iodine solution. Amylopectin, on the other hand, does not give blue color with iodine solution.
Structure of amylose: It is a linear polymer of \[\alpha -D-glu\cos e\] in which\[{{C}_{1}}\] of one glucose unit is attached to\[{{C}_{4}}\] of the other through\[\alpha -gly\cos idic\] linkage.

Structure of amylopectin: Amylopectin, on the other hand, is a highly branched polymer. It consists of a large number of short chains each containing 20-25 glucose units which are joined together through \[\alpha -gly\cos idic\] linkage involving \[{{C}_{1}}\] of one glucose unit with\[{{C}_{4}}\] of the other. The \[{{C}_{1}}\]of the terminal glucose unit in each chain is further linked to\[{{C}_{6}}\] of some other glucose unit in the next chain through \[{{C}_{1}}-{{C}_{6}}\]\[\alpha -gly\cos idic\] linkage. This gives amylopectin a highly branched structure.

Hence, from the above discussion option (b) is correct.
Note: You may get confused between \[\alpha -linkage\] and \[\beta -linkage\]. \[\alpha -linkage\] is formed when the bond is formed on the same side and \[\beta -linkage\]is formed when the bond is formed between the opposite side.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Understanding the Angle of Deviation in a Prism

Inductive Effect and Its Role in Acidic Strength

Understanding Average and RMS Value in Electrical Circuits

Other Pages
JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

CBSE Notes Class 12 Chemistry Chapter 3 - Chemical Kinetics - 2025-26

What is Glucose in Chemistry? Structure, Properties & Uses

JEE Main Correction Window 2026 Session 1 Dates Announced - Edit Form Details, Dates and Link

CBSE Notes Class 12 Chemistry Chapter 9 - Amines - 2025-26

How will you convert i Propene to propan1ol ii Ethanal class 12 chemistry JEE_Main




