
Structure formula of \[{H_2}{O_2}\] is
(a) 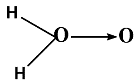
(b) \[H - O - O - H\](Straight line).
(c) Where, \[\angle H - O - O = \angle O - O - H' = {101.5^o}\]and all the four atoms are in the same plane. 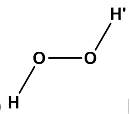
(d) Where, \[\angle H - O - O = \angle O - O - H' = {97^o}\]and the angle between \[H - O - O\]plane and \[O - O - H'\]plane is \[{101^o}\]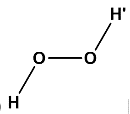
Answer
240.9k+ views
Hint: Hydrogen peroxide (\[{H_2}{O_2}\]) is the type of simplest known peroxide with oxygen-oxygen single bond. It has a non-planar and open book-type structure. Depending upon the reaction medium, it can behave like an oxidizing and reducing agent.
Complete step by step solution:Hydrogen peroxide is like water with an extra oxygen atom. Due to the presence of \[ - OH\] groups, it has an acidic nature( \[pH = 4.5\]).
Hydrogen peroxide is \[100\% \]degradable compound.
Hydrogen peroxide is considered as non-planar in nature with an open book-like structure.
The structure of hydrogen peroxide indicates the presence of an oxygen-oxygen single bond( \[O - O\]) with bond length \[145.5pm\]. Whereas the \[O - H\]bond length is around \[98.8pm\].
The non-planar open-book structure of hydrogen peroxide has two planes and both the planes contain \[OH\]group. The bond angle between both the planes is \[{90.2^o}\].
The bond angle between the \[H - O - O\]is \[101.{9^o}\].
The open-book structure of hydrogen peroxide can be represented as below.
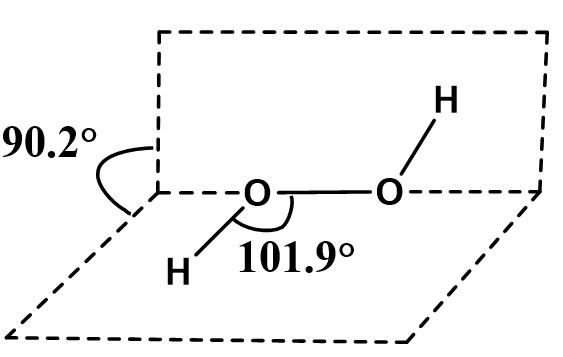
Image: An open-book structure of hydrogen peroxide.
Therefore from the above explanation we can say option (d) will be the correct option:
Note: The purest form of hydrogen peroxide is colorless in nature.
Hydrogen peroxide melts at \[272.4K\]and boils at \[423K\].
Hydrogen peroxide is miscible in water in all ratios.
The oxygen atom has -1 oxidation state in hydrogen peroxide.
Hydrogen peroxide is also used as a bleaching agent.
Complete step by step solution:Hydrogen peroxide is like water with an extra oxygen atom. Due to the presence of \[ - OH\] groups, it has an acidic nature( \[pH = 4.5\]).
Hydrogen peroxide is \[100\% \]degradable compound.
Hydrogen peroxide is considered as non-planar in nature with an open book-like structure.
The structure of hydrogen peroxide indicates the presence of an oxygen-oxygen single bond( \[O - O\]) with bond length \[145.5pm\]. Whereas the \[O - H\]bond length is around \[98.8pm\].
The non-planar open-book structure of hydrogen peroxide has two planes and both the planes contain \[OH\]group. The bond angle between both the planes is \[{90.2^o}\].
The bond angle between the \[H - O - O\]is \[101.{9^o}\].
The open-book structure of hydrogen peroxide can be represented as below.

Image: An open-book structure of hydrogen peroxide.
Therefore from the above explanation we can say option (d) will be the correct option:
Note: The purest form of hydrogen peroxide is colorless in nature.
Hydrogen peroxide melts at \[272.4K\]and boils at \[423K\].
Hydrogen peroxide is miscible in water in all ratios.
The oxygen atom has -1 oxidation state in hydrogen peroxide.
Hydrogen peroxide is also used as a bleaching agent.
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Difference Between Crystalline and Amorphous Solid: Table & Examples

Types of Solutions in Chemistry: Explained Simply

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

Trending doubts
Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Inductive Effect and Its Role in Acidic Strength

JEE Main Correction Window 2026 Session 1 Dates Announced - Edit Form Details, Dates and Link

Other Pages
JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

Iodine is a nonmetal which has metallic luster A True class 11 chemistry JEE_Main

Understanding Collisions: Types and Examples for Students

Free Radical Substitution and Its Stepwise Mechanism

How Does Fusion Reaction Happen Inside the Sun?

JEE Main 2026 Helpline Numbers for Aspiring Candidates




