
Select correct statement for $Br{F_5}.$
(A) All fluorine atoms are in the same plane.
(B) Four fluorine atoms and Br atoms are in the same plane.
(C) Four fluorine atoms are in the same plane.
(D) It has\[F - Br - F\]bond angles at ${90^\circ }.$
Answer
232.5k+ views
Hint: $Br{F_5}$ is an interhalogen compound. When two different halogens form compounds among themselves, interhalogen compounds are formed. Such compound may have general formula $X{X^1},XX_3^1,XX_5^1$ and $XX_7^1.$ Here $X$ is halogen of large size and ${X^1}$of small size and $X$ is more negative.
Complete step by step answer:
In $Br{F_5}$Bromine is the central atom and fluorine atoms present around it.
The name of the compound is Bromine pentafluoride.
Bromine atoms have larger size than fluorine and fluorine is more electronegative.
Electronic configuration of Br, $1{s^2}2{s^2}2{p^6}2{s^2}3{p^6}3{d^{10}}4{s^2}4p_x^24p_y^24p_z^1$
Ground state configuration =
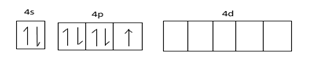
Excited state configuration =
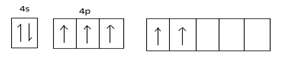
Hybridized state =
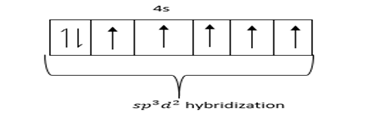
Geometry of $Br{F_5}$ is square pyramidal and hybridization $s{p^3}{d^2}.$
In $s{p^3}{d^2},$ 4 Fluorine atoms and one Br-atom present in the same plane.
Bond angle between them is 90 Degrees.
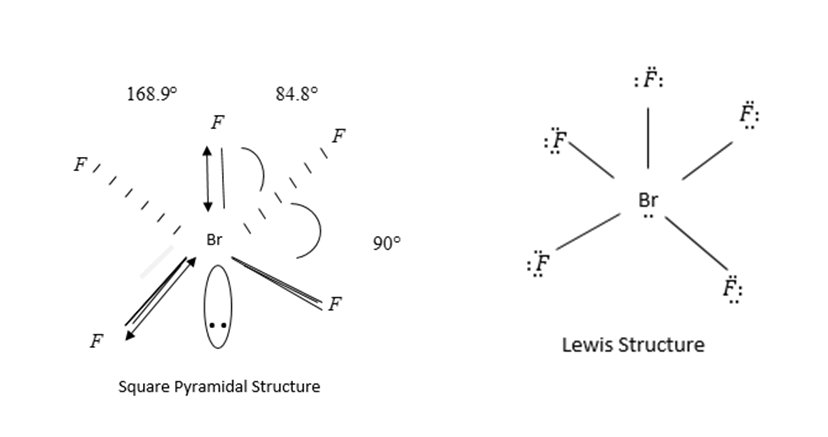
In Lewis structure we see that octets of each element should be complete. On fluorine atoms 3 lone pairs of electrons and on bromine atom only one lone pair is present.
By VSEPR theory 4 bond pairs occupy corners of squares and one bond pair and lone pair of electrons occupy equatorial position to minimize repulsion between lone pair and bond pair of electron.
Interhalogen compounds are covalent in nature due to low electronegativity difference between halogens. They are diamagnetic in nature. They are more reactive than halogen.
Interhalogen compounds behave as strong oxidizing agents and attack other elements to give a mixture of halides.
Therefore, the above explanation, the correct option is [D] Both assertion and reason are incorrect.
Note: Interhalogen compounds are more reactive than halogen. This is because the $X - {X^ - }$ bond present in them is weaker than $X - X$ and${X^1} - X$ bonds.
Complete step by step answer:
In $Br{F_5}$Bromine is the central atom and fluorine atoms present around it.
The name of the compound is Bromine pentafluoride.
Bromine atoms have larger size than fluorine and fluorine is more electronegative.
Electronic configuration of Br, $1{s^2}2{s^2}2{p^6}2{s^2}3{p^6}3{d^{10}}4{s^2}4p_x^24p_y^24p_z^1$
Ground state configuration =

Excited state configuration =

Hybridized state =

Geometry of $Br{F_5}$ is square pyramidal and hybridization $s{p^3}{d^2}.$
In $s{p^3}{d^2},$ 4 Fluorine atoms and one Br-atom present in the same plane.
Bond angle between them is 90 Degrees.

In Lewis structure we see that octets of each element should be complete. On fluorine atoms 3 lone pairs of electrons and on bromine atom only one lone pair is present.
By VSEPR theory 4 bond pairs occupy corners of squares and one bond pair and lone pair of electrons occupy equatorial position to minimize repulsion between lone pair and bond pair of electron.
Interhalogen compounds are covalent in nature due to low electronegativity difference between halogens. They are diamagnetic in nature. They are more reactive than halogen.
Interhalogen compounds behave as strong oxidizing agents and attack other elements to give a mixture of halides.
Therefore, the above explanation, the correct option is [D] Both assertion and reason are incorrect.
Note: Interhalogen compounds are more reactive than halogen. This is because the $X - {X^ - }$ bond present in them is weaker than $X - X$ and${X^1} - X$ bonds.
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Types of Solutions in Chemistry: Explained Simply

Difference Between Crystalline and Amorphous Solid: Table & Examples

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE General Topics in Chemistry Important Concepts and Tips

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




