
$Sb{{F}_{5}}$ reacts with $Xe{{F}_{4}}$ and $Xe{{F}_{6}}$ to form ionic compounds $\left[ XeF_{3}^{+} \right]\left[ SbF_{6}^{-} \right]$ and $\left[ XeF_{5}^{+} \right]\left[ SbF_{6}^{-} \right]$. The geometry of $XeF_{3}^{+}$, $\left[ XeF_{5}^{+} \right]$ ion respectively is:
(A) square pyramidal, T-shaped
(B) bent T-shaped, square pyramidal
(C) see-saw, square pyramidal
(D) square pyramidal, see-saw
Answer
233.1k+ views
Hint: By obtaining the hybridisation of the central metal atom with respect to the ligands by considering the oxidation state and the configuration of the central atom. Then, from the lone pairs and the bond pairs, the geometry can be determined.
Complete step by step solution:
It is given that the $Sb{{F}_{5}}$ reacts with $Xe{{F}_{4}}$ and $Xe{{F}_{6}}$, acting as a Lewis acid as follows :
\[Xe{{F}_{4}}+Sb{{F}_{5}}\to {{\left[ Xe{{F}_{3}} \right]}^{+}}{{\left[ Sb{{F}_{6}} \right]}^{-}}\]
$Xe{{F}_{6}}+Sb{{F}_{5}}\to {{\left[ Xe{{F}_{5}} \right]}^{+}}{{\left[ Sb{{F}_{6}} \right]}^{-}}$
Now, in order to find the geometry of the cationic sphere, we will use the VSEPR theory as follows:
-In both the cations, xenon being least electronegative, is the central atom. Its electronic configuration at ground state is $\left[ Kr \right]4{{d}^{10}}5{{s}^{2}}5{{p}^{6}}$.
-Due to the presence of a $(+1)$ charge on the sphere, that is, loss of an electron, the xenon loses one of its electrons from the 5p orbital. So, the configuration of $X{{e}^{+}}$ becomes $\left[ Kr \right]4{{d}^{10}}5{{s}^{2}}5{{p}^{5}}$.
-In case of $XeF_{3}^{+}$, in order to form three bonds with the fluorine atom. The xenon atom goes into an excited state and one of its 5p electrons jumps to 5d orbital. Now, these 5s, 5p and 5d-orbitals undergo hybridisation to form five $s{{p}^{3}}d$ hybrid orbitals, consisting of two lone pairs and three unpaired electrons.
-These unpaired electrons in the three hybrid orbitals overlap with an unpaired electron present in the three-fluorine p-orbital, one in each.
Thus, the hybridisation obtained in order to form $XeF_{3}^{+}$is $s{{p}^{3}}d$ hybridisation. So, the geometry will be trigonal bipyramidal, with one bond pair and two lone pairs in the equatorial position and the other two bond pairs in the axial position.
Due to the repulsion from the two lone pairs, we get a bent T-shaped molecule.
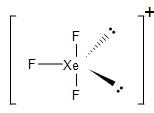
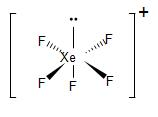
Similarly, in the case of $XeF_{5}^{+}$, in order to form five bond pairs with the fluorine atom. In the excited state, two p-electrons jump to the d-orbital. the 5s, 5p and 5d orbitals undergo hybridisation to form six $s{{p}^{3}}{{d}^{2}}$ hybrid orbitals. Thus, generating one lone pair and five unpaired electrons.
The five unpaired electrons overlap with the fluorine p-orbitals and form five bond pairs. Therefore, we get $s{{p}^{3}}{{d}^{2}}$ hybridisation and giving us an octahedral geometry. Due to the presence of the lone pair, its geometry gets distorted and we get a square pyramidal molecule.
Therefore, the shape of the $XeF_{3}^{+}$and $XeF_{5}^{+}$ entity is option (B)- bent T-shaped, square pyramidal.
Note: In order to obtain the hybridisation, the charge on the ion must be taken to obtain the oxidation state of the metal atom and then we would arrive at the appropriate hybridisation.
Complete step by step solution:
It is given that the $Sb{{F}_{5}}$ reacts with $Xe{{F}_{4}}$ and $Xe{{F}_{6}}$, acting as a Lewis acid as follows :
\[Xe{{F}_{4}}+Sb{{F}_{5}}\to {{\left[ Xe{{F}_{3}} \right]}^{+}}{{\left[ Sb{{F}_{6}} \right]}^{-}}\]
$Xe{{F}_{6}}+Sb{{F}_{5}}\to {{\left[ Xe{{F}_{5}} \right]}^{+}}{{\left[ Sb{{F}_{6}} \right]}^{-}}$
Now, in order to find the geometry of the cationic sphere, we will use the VSEPR theory as follows:
-In both the cations, xenon being least electronegative, is the central atom. Its electronic configuration at ground state is $\left[ Kr \right]4{{d}^{10}}5{{s}^{2}}5{{p}^{6}}$.
-Due to the presence of a $(+1)$ charge on the sphere, that is, loss of an electron, the xenon loses one of its electrons from the 5p orbital. So, the configuration of $X{{e}^{+}}$ becomes $\left[ Kr \right]4{{d}^{10}}5{{s}^{2}}5{{p}^{5}}$.
-In case of $XeF_{3}^{+}$, in order to form three bonds with the fluorine atom. The xenon atom goes into an excited state and one of its 5p electrons jumps to 5d orbital. Now, these 5s, 5p and 5d-orbitals undergo hybridisation to form five $s{{p}^{3}}d$ hybrid orbitals, consisting of two lone pairs and three unpaired electrons.
-These unpaired electrons in the three hybrid orbitals overlap with an unpaired electron present in the three-fluorine p-orbital, one in each.
Thus, the hybridisation obtained in order to form $XeF_{3}^{+}$is $s{{p}^{3}}d$ hybridisation. So, the geometry will be trigonal bipyramidal, with one bond pair and two lone pairs in the equatorial position and the other two bond pairs in the axial position.
Due to the repulsion from the two lone pairs, we get a bent T-shaped molecule.


Similarly, in the case of $XeF_{5}^{+}$, in order to form five bond pairs with the fluorine atom. In the excited state, two p-electrons jump to the d-orbital. the 5s, 5p and 5d orbitals undergo hybridisation to form six $s{{p}^{3}}{{d}^{2}}$ hybrid orbitals. Thus, generating one lone pair and five unpaired electrons.
The five unpaired electrons overlap with the fluorine p-orbitals and form five bond pairs. Therefore, we get $s{{p}^{3}}{{d}^{2}}$ hybridisation and giving us an octahedral geometry. Due to the presence of the lone pair, its geometry gets distorted and we get a square pyramidal molecule.
Therefore, the shape of the $XeF_{3}^{+}$and $XeF_{5}^{+}$ entity is option (B)- bent T-shaped, square pyramidal.
Note: In order to obtain the hybridisation, the charge on the ion must be taken to obtain the oxidation state of the metal atom and then we would arrive at the appropriate hybridisation.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




