
\({\rm{C}}{{\rm{H}}_{\rm{2}}} = {\rm{C}}{{\rm{H}}_{\rm{2}}} \overset{Alkaline KMnO_{4},KOH/H_{2}O}{\rightarrow} X\). Product X in the above reaction is [RPMT 2003]
A) Ethylene glycol
B) Glucose
C) Ethanol
D) All of these
Answer
233.1k+ views
Hint: We know that potassium permanganate is an oxidising agent (strong). It is used in a set of chemical reactions. Here, we have to identify the product obtained on the reaction ethene with potassium permanganate in the basic medium.
Complete Step by Step Answer:
We know that the strong oxidising nature of potassium permanganate gives oxygen in an alkaline or neutral medium. The oxygen obtained has a role in the oxidation of alkene to form 1,2-diol. Therefore, the reaction of ethene with the potassium permanganate in the alkaline medium gives the product of ethylene glycol or 1,2-diol.
The oxidation reaction is as follows:
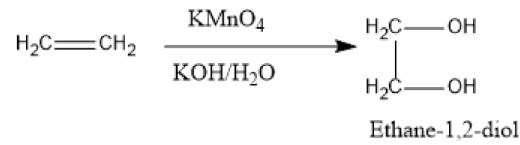
Image: Reaction of ethene with potassium permanganate in alkaline medium
Let's understand some facts regarding the above reaction. The reaction is also termed hydroxylation reaction because of the attachment of the hydroxyl groups across the double bond of the ethene. In this reaction, the potassium permanganate loses its pink colour and a precipitate of manganese dioxide is obtained. This test is termed Baeyer's test. Therefore, product X in the reaction is ethylene glycol.
Hence, option A is right.
Note: The cold, acidified and potassium permanganate(dilute) is termed Baeyer's reagent. It is useful in determining alkenes and alkynes. And in this test, if the decolourisation of potassium permanganate happens, confirms the unsaturated nature of the compounds. An alkene when undergoes a reaction with cold, acidified and dilute potassium permanganate gives vicinal diols.
Complete Step by Step Answer:
We know that the strong oxidising nature of potassium permanganate gives oxygen in an alkaline or neutral medium. The oxygen obtained has a role in the oxidation of alkene to form 1,2-diol. Therefore, the reaction of ethene with the potassium permanganate in the alkaline medium gives the product of ethylene glycol or 1,2-diol.
The oxidation reaction is as follows:
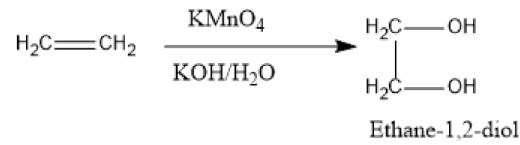
Image: Reaction of ethene with potassium permanganate in alkaline medium
Let's understand some facts regarding the above reaction. The reaction is also termed hydroxylation reaction because of the attachment of the hydroxyl groups across the double bond of the ethene. In this reaction, the potassium permanganate loses its pink colour and a precipitate of manganese dioxide is obtained. This test is termed Baeyer's test. Therefore, product X in the reaction is ethylene glycol.
Hence, option A is right.
Note: The cold, acidified and potassium permanganate(dilute) is termed Baeyer's reagent. It is useful in determining alkenes and alkynes. And in this test, if the decolourisation of potassium permanganate happens, confirms the unsaturated nature of the compounds. An alkene when undergoes a reaction with cold, acidified and dilute potassium permanganate gives vicinal diols.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




