
Question:
The IUPAC name of chloretone is:
(A) Trichloroacetone
(B) Trichloronitromethane
(C) 1, 1, 1-trichloro-2-methyl-2-propanone
(D) 1, 1, 1-trichloro-2-methyl-2-propanol
Answer
233.1k+ views
Hint: Chloretone has a hydroxyl group and a halide (chlorine) as a functional group. We can use –ol suffix to describe hydroxyl functional groups and use Halo- prefix to describe presence of halogen in IUPAC nomenclature.
Step by step answer:
First of all we should know about chloretone. Chloretone is also called chlorobutanol, or chlorbutol.
- It is an alcohol based preservative. We use it as a preservative, sedative, hypnotic and weak local anesthetic similar in nature to chloral hydrate. It has antibacterial and antifungal properties. Now, we will draw the structure of chloretone.
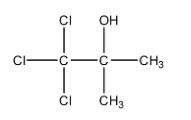
Let’s try to give the IUPAC name to this compound.
- To name this compound, we will first need to identify the longest carbon chain and we can see that there is a three carbon chain possible. We will start numbering the chain from the carbon that has a halogen group.
- There are 3 chlorine groups present at the first carbon chain and there is one alcohol group and one methyl group present on the second carbon of the chain. We will use –ol suffix to represent hydroxyl groups.
- So, we can say that the IUPAC name of chloretone is, 1, 1, 1- trichloro-2-methyl-2-propanol.
By this we can say that, option D is correct.
Note: Do not forget to arrange the substituent groups on the main carbon chain according to the alphabetical order in the process of naming a compound. Here, three chlorine atoms are present at the first carbon atom of the chain, so we will use trichloro- prefix instead of mentioning them separately.
Step by step answer:
First of all we should know about chloretone. Chloretone is also called chlorobutanol, or chlorbutol.
- It is an alcohol based preservative. We use it as a preservative, sedative, hypnotic and weak local anesthetic similar in nature to chloral hydrate. It has antibacterial and antifungal properties. Now, we will draw the structure of chloretone.

Let’s try to give the IUPAC name to this compound.
- To name this compound, we will first need to identify the longest carbon chain and we can see that there is a three carbon chain possible. We will start numbering the chain from the carbon that has a halogen group.
- There are 3 chlorine groups present at the first carbon chain and there is one alcohol group and one methyl group present on the second carbon of the chain. We will use –ol suffix to represent hydroxyl groups.
- So, we can say that the IUPAC name of chloretone is, 1, 1, 1- trichloro-2-methyl-2-propanol.
By this we can say that, option D is correct.
Note: Do not forget to arrange the substituent groups on the main carbon chain according to the alphabetical order in the process of naming a compound. Here, three chlorine atoms are present at the first carbon atom of the chain, so we will use trichloro- prefix instead of mentioning them separately.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




