
Product obtained by electrolytic reduction of nitrobenzene in presence of \[{H_2}S{O_4}\] is
A. o-amino phenol
B. m-amino phenol
C. p-amino phenol
D. None of these
Answer
232.8k+ views
Hint: Electrolytic reduction is a method where electric current is passed through the ionic solution leading to chemical reaction at the electrodes. Strong acids like hydrochloric acid and sulphuric acid are used as an acidic medium.
Complete Step by Step Solution:
First we should know about electrolytic reduction. It is a method where electric current is passed through an electrolyte and a chemical reaction takes place in the two electrodes i.e, anode and cathodes and the compound decomposes to form a new product. The reaction takes place in an acidic medium. In this reaction the acidic medium used is sulphuric acid.
Nitrobenzene is an oily aromatic compound where a nitro group (nitrogen dioxide) replaces the hydrogen atom of the benzene ring.
The electrolytic reduction reaction of nitrobenzene in presence of sulphuric acid is shown below.
First nitrobenzene in the presence of an acidic medium undergoes electrolytic reduction to form phenyl hydroxylamine as a product.
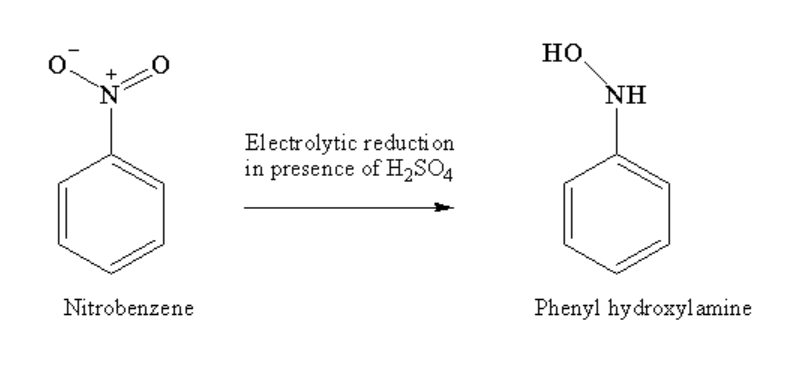
Image: Electrolytic reduction of nitrobenzene
Phenylhydroxylamine further undergoes rearrangement to form p-Amino phenol as the main product.
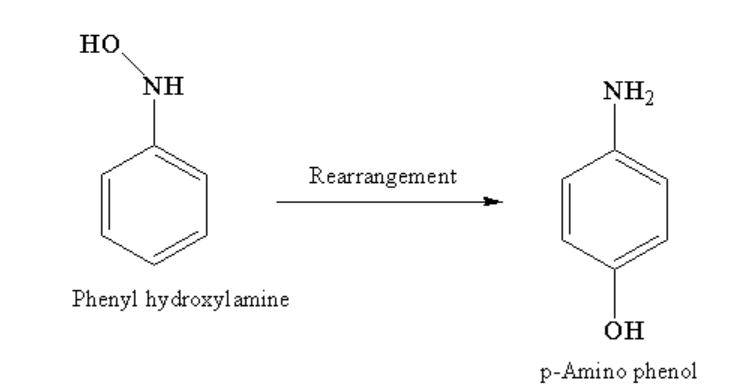
Image: Rearrangement
Therefore, the correct option is C. .
Note: It should be noted that nitrobenzene on electrolytic reduction in presence of weak acidic medium forms aniline as a product but in case of strong acidic medium it forms p-aminophenol. In alkaline medium, mono and di- nuclear reduction products are formed such as azoxybenzene and azobenzene.
Complete Step by Step Solution:
First we should know about electrolytic reduction. It is a method where electric current is passed through an electrolyte and a chemical reaction takes place in the two electrodes i.e, anode and cathodes and the compound decomposes to form a new product. The reaction takes place in an acidic medium. In this reaction the acidic medium used is sulphuric acid.
Nitrobenzene is an oily aromatic compound where a nitro group (nitrogen dioxide) replaces the hydrogen atom of the benzene ring.
The electrolytic reduction reaction of nitrobenzene in presence of sulphuric acid is shown below.
First nitrobenzene in the presence of an acidic medium undergoes electrolytic reduction to form phenyl hydroxylamine as a product.

Image: Electrolytic reduction of nitrobenzene
Phenylhydroxylamine further undergoes rearrangement to form p-Amino phenol as the main product.

Image: Rearrangement
Therefore, the correct option is C. .
Note: It should be noted that nitrobenzene on electrolytic reduction in presence of weak acidic medium forms aniline as a product but in case of strong acidic medium it forms p-aminophenol. In alkaline medium, mono and di- nuclear reduction products are formed such as azoxybenzene and azobenzene.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




