




How Did Rutherford’s Experiment Change Our View of the Atom?
Alpha particle scattering and the Rutherford model of atom are essential topics in atomic physics. These concepts provide the basis for understanding atomic structure and the discovery of the atomic nucleus, which are foundational for JEE Main and Advanced Physics preparation.
Background of Alpha Particle Scattering Experiment
The alpha particle scattering experiment investigated the internal structure of atoms using a beam of alpha particles. This classic experiment was carried out by Ernest Rutherford, Hans Geiger, and Ernest Marsden in 1911 to probe the arrangement of mass and charge within atoms.
Alpha particles are helium nuclei with a $+2e$ charge and significant mass. They were ideal probes due to their high kinetic energy and positive charge, which allowed their trajectories to be altered by the electric fields within the atom.
The experiment is discussed in detail in other contexts such as Alpha, Beta, And Gamma Decay where different types of radioactive emissions are analyzed.
Experimental Setup and Methodology
The experimental setup consisted of a thin gold foil, a source of alpha particles, a lead shield with a small aperture, and a circular fluorescent screen coated with zinc sulphide. The screen detected scattered alpha particles by producing visible flashes upon impact.
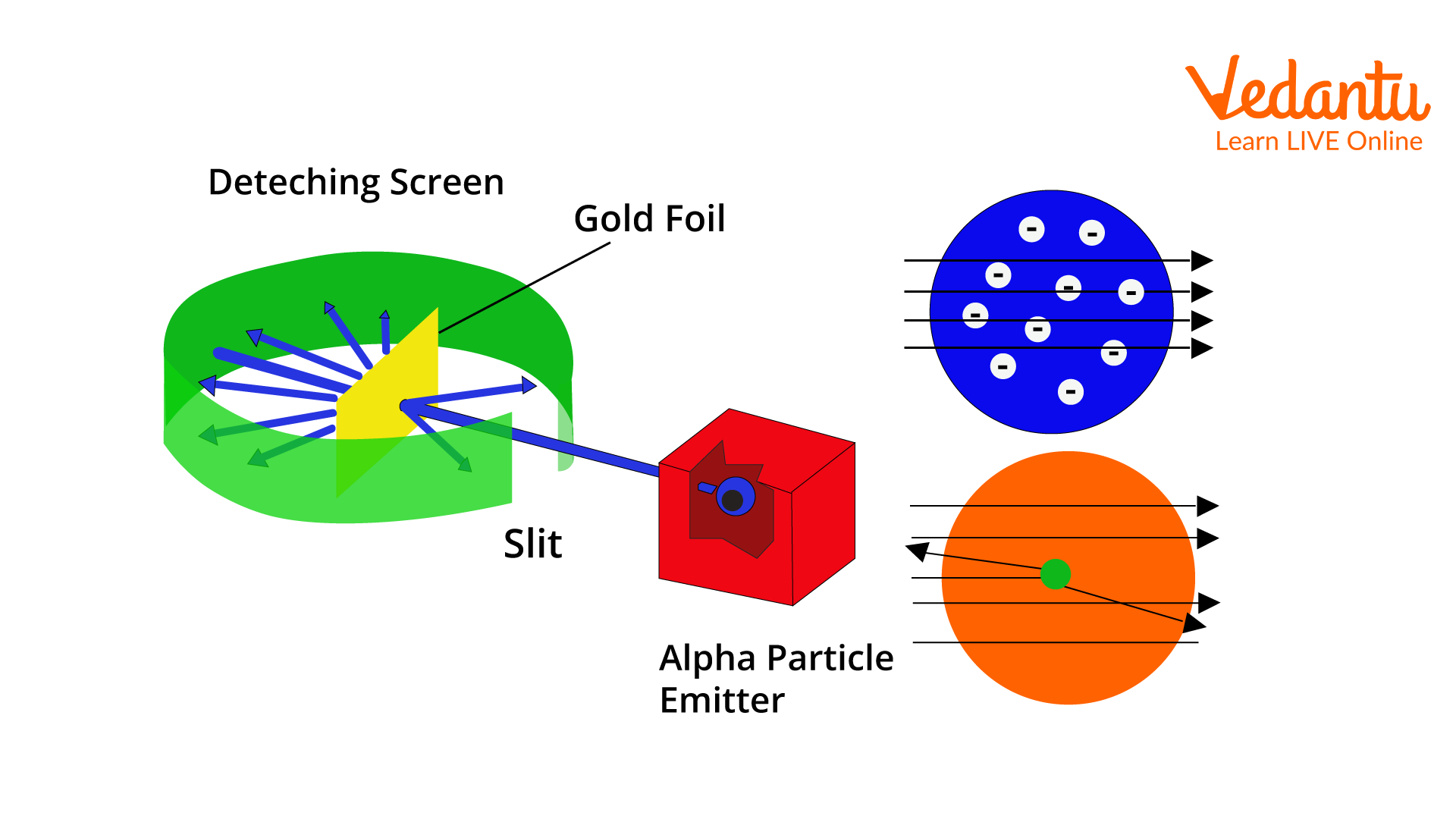
A beam of alpha particles was directed at the thin gold foil. Most particles passed straight through, some were deflected at small angles, and a few were scattered at angles larger than $90^\circ$, including near reversal of direction.
Key Observations from the Experiment
The results of the alpha particle scattering experiment revealed the following important points about atomic structure:
- Most alpha particles passed straight with no deflection
- Some were deflected at small angles
- Very few were deflected at large angles
- Almost none were reflected back
Such observations implied the presence of vast empty space within atoms, a small, dense region with positive charge, and the need for a new atomic model.
The understanding of how atomic models evolved is further addressed in Atomic Structure where comparisons of different atomic models are made.
Rutherford’s Nuclear Model of the Atom
Based on the observations, Rutherford proposed that atoms contain a small, massive, positively charged nucleus at the center. Electrons revolve around this nucleus in orbits, and the majority of atomic volume is empty space.
The positive charge and nearly all atomic mass reside in the nucleus. The electrostatic attraction between the nucleus and electrons holds the atom together. This model marked a major advancement from the earlier Thomson’s model.
| Feature | Rutherford Model |
|---|---|
| Nucleus present | Yes, small and positively charged |
| Electrons | Revolve in orbits |
| Atomic mass location | Concentrated at nucleus |
| Empty space | Most of the atom |
Alpha Particle Scattering: Quantitative Analysis
Rutherford derived a formula to describe the probability of alpha particles scattering at different angles. The scattering angle $\theta$ is related to the impact parameter and the electric force between the positively charged nucleus and alpha particle.
The number of alpha particles $N(\theta)$ scattered at an angle $\theta$ is given by:
$N(\theta) \propto \dfrac{1}{(\sin^4 \dfrac{\theta}{2})}$
Large angle deflections are rare because the nucleus is small, so only alpha particles passing very close to the center experience strong repulsion. This supported a compact nucleus model.
The properties of alpha, beta, and gamma rays, which relate to the types of radioactive emissions used in similar experiments, can be found at Properties Of Alpha, Beta, And Gamma Rays.
Why Alpha Particles Were Used
Alpha particles were used because they are relatively heavy, positively charged, and stable. Their large mass and double positive charge increased the likelihood of observable deflection by the atomic nucleus, suitable for investigating atomic structure.
Limitations of Rutherford’s Nuclear Model
Despite its success, Rutherford’s model could not explain the stability of atoms. According to classical electromagnetic theory, revolving electrons should radiate energy and spiral into the nucleus, leading to atomic collapse, which does not occur in reality.
The model also did not address electron arrangement or atomic spectra. These issues motivated further investigation, leading to the Bohr model and subsequent quantum theories.
Detailed studies of nuclear processes, including fission and fusion, are presented at Nuclear Fission And Fusion.
Key Takeaways from the Gold Foil Experiment
- Atom is mostly empty space
- Nucleus hosts all positive charge and mass
- Electrons move around the nucleus
- Classical mechanics cannot fully explain atomic stability
Further aspects of atomic and nuclear physics, including applications and advanced concepts, can be explored in Atom And Nuclei.
Summary of Rutherford Alpha Particle Scattering
The Rutherford alpha particle scattering experiment demonstrated that the atom consists of a tiny, dense, positively charged nucleus surrounded by electrons. The findings provided vital evidence for the current understanding of atomic structure, although the explanation for atomic stability required further development.
The experimental approach and quantitative analysis continue to serve as essential milestones in the study of atomic and nuclear physics, forming the basis for further advancements in modern science and the JEE Main syllabus.
FAQs on Understanding Alpha Particle Scattering and the Rutherford Model of Atom
1. What was the main conclusion of Rutherford’s alpha particle scattering experiment?
Rutherford's alpha particle scattering experiment concluded that most of the atom’s mass and its positive charge are concentrated in a small core called the nucleus.
- Most alpha particles passed through the gold foil undeflected, indicating atoms are mostly empty space.
- A few particles were deflected at large angles, showing the presence of a small, dense, positively charged center.
- This discovery led to the formulation of the Rutherford model of the atom, replacing earlier atomic models.
2. How did Rutherford’s model of the atom differ from Thomson’s model?
Rutherford's atomic model proposed a dense atomic nucleus, while Thomson’s model was known as the ‘plum pudding model’.
- Rutherford: Atom has a central nucleus containing positive charge; electrons move around it.
- Thomson: Atom is a positively charged sphere with embedded electrons.
- Rutherford’s model explained the atom’s structure based on alpha particle scattering results, unlike Thomson’s model.
3. What are the major observations from the alpha particle scattering experiment?
The key observations from the alpha particle scattering experiment are:
- Most alpha particles passed straight through the gold foil.
- Some particles were deflected by small angles.
- A very few alpha particles bounced back.
These findings showed that atoms have a tiny, dense nucleus and are mostly empty space.
4. What are the main features of Rutherford’s atomic model?
Rutherford’s atomic model described the atom as mostly empty space with electrons revolving around a central nucleus.
- Atom has a dense, positively charged nucleus at its center.
- Electrons revolve in orbits around the nucleus.
- The nucleus contains almost all the mass of the atom.
This model explained atomic structure based on experimental evidence.
5. What were the limitations of Rutherford’s model of the atom?
Limitations of Rutherford’s atomic model included inability to explain electron stability in fixed orbits.
- Did not explain why electrons don’t spiral into the nucleus despite attraction.
- Could not describe the stability of atoms.
- Failed to explain the discrete lines in atomic spectra.
This led to the development of the Bohr model of the atom.
6. Why was a gold foil used in the alpha particle scattering experiment?
A thin gold foil was used in the alpha scattering experiment because gold is highly malleable and can be made extremely thin.
- Its thinness allowed most alpha particles to pass through with minimal obstruction.
- Gold has a high atomic number, which makes the scattering effect more observable.
It helped reveal the atom’s internal structure effectively.
7. What does the alpha particle scattering experiment tell us about the structure of the atom?
The alpha particle scattering experiment shows that atoms have a tiny, central, positively charged nucleus and are mostly empty space.
- The nucleus contains nearly all the atom's mass.
- Electrons move around the nucleus.
- Most of the atom is empty space, allowing alpha particles to pass through.
This experiment redefined our understanding of atomic structure.
8. Who discovered the nucleus and how?
Ernest Rutherford discovered the atomic nucleus in 1911 through the alpha particle scattering experiment.
- Rutherford observed some alpha particles reflected at large angles.
- He concluded that a dense, positively charged center existed in the atom.
This led to the identification of the nucleus as the core of the atom.
9. State the postulates of Rutherford’s atomic model.
Rutherford’s atomic model is based on these main postulates:
1. The atom has a small, dense, positively charged nucleus at its center.
2. Electrons revolve around the nucleus in defined orbits.
3. Most of the atom's volume is empty space.
These ideas explained atomic structure and led to advanced atomic models.
10. Explain why most alpha particles passed through the gold foil undeflected.
Most alpha particles passed undeflected because atoms are mostly empty space.
- Only a tiny, dense nucleus deflected a few particles.
- The vast space around the nucleus had no obstructions for the alpha particles.
This observation supported the nuclear model of the atom.
11. What is the significance of the alpha particle scattering experiment for modern atomic theory?
The alpha particle scattering experiment is highly significant because it established the concept of the nucleus and led to the modern atomic structure.
- It proved the existence of a small, dense nucleus.
- It disproved the earlier plum pudding model.
- It provided the experimental basis for the Rutherford model and later, the Bohr model.
This experiment transformed atomic physics and chemistry.


































