
One mole of each of the following alkenes is catalytically hydrogenated. The quantity of heat evolved will be the lowest in the case of
A) 1-butene
B) Trans-2-butene
C) Cis-2-butene
D) 1,3-butadiene
Answer
233.1k+ views
Hint: The hydrogenation reaction defines the reaction of molecular hydrogen with an alkene in presence of catalysts like nickel, palladium, etc. Here, we have to compare the heat released in the hydrogenation of the given alkenes.
Complete Step by Step Answer:
Let's understand what the heat of hydrogenation is. This is the quantity of heat that evolved in a hydrogenation reaction. The stability of the alkenes has an impact on the release of heat. The more the alkene's stability, the less the amount of heat released.
Now, look at the options. Here, we have to find symmetrical molecules because they are highly stable.
1-butene is not a symmetrical alkene. So, it has a high heat of hydrogenation. So, option A is wrong.
1,3-butadiene is also not a symmetrical alkene. So, option D is also wrong.
Now, the remaining alkenes are symmetrical alkenes. They are, trans-2-butene and cis-2-butene.
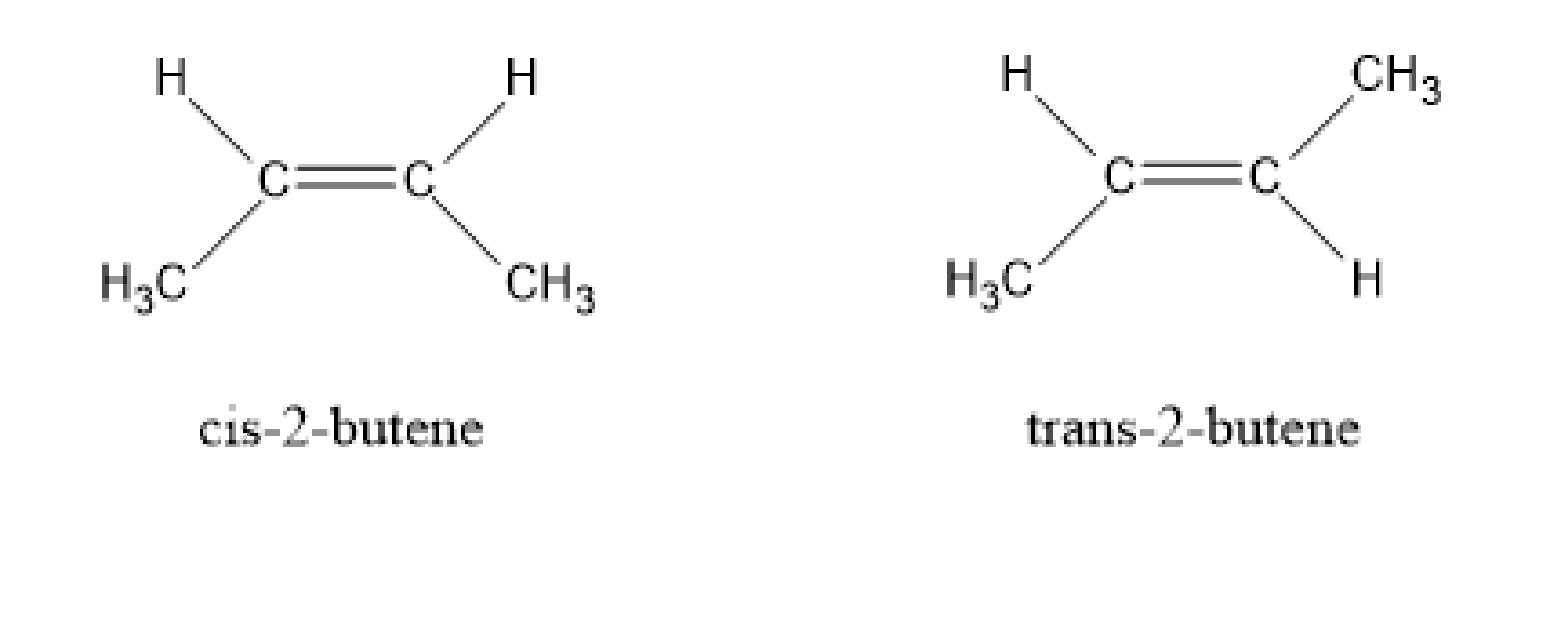
Image: cis-2-butene and trans-2-butene
As we find that, both these alkenes are symmetrical. Now, we have to compare the heat released in the hydrogenation of these two alkenes. The stability of trans-2-butene is more than cis-2-butene because, in the former, the methyl groups are at the distant positions but in the latter, the methyl groups are in the crowded position that causes steric repulsion. So, the heat of hydrogenation is the least in the case of trans-2-butene.
Hence, option B is right.
Note: It is to be noted that, the direct conversion of an alkene to alkane happens by the addition of hydrogen in presence of catalysts like nickel. The hydrogenation of alkynes can be done by using Lindlar's catalyst.
Complete Step by Step Answer:
Let's understand what the heat of hydrogenation is. This is the quantity of heat that evolved in a hydrogenation reaction. The stability of the alkenes has an impact on the release of heat. The more the alkene's stability, the less the amount of heat released.
Now, look at the options. Here, we have to find symmetrical molecules because they are highly stable.
1-butene is not a symmetrical alkene. So, it has a high heat of hydrogenation. So, option A is wrong.
1,3-butadiene is also not a symmetrical alkene. So, option D is also wrong.
Now, the remaining alkenes are symmetrical alkenes. They are, trans-2-butene and cis-2-butene.

Image: cis-2-butene and trans-2-butene
As we find that, both these alkenes are symmetrical. Now, we have to compare the heat released in the hydrogenation of these two alkenes. The stability of trans-2-butene is more than cis-2-butene because, in the former, the methyl groups are at the distant positions but in the latter, the methyl groups are in the crowded position that causes steric repulsion. So, the heat of hydrogenation is the least in the case of trans-2-butene.
Hence, option B is right.
Note: It is to be noted that, the direct conversion of an alkene to alkane happens by the addition of hydrogen in presence of catalysts like nickel. The hydrogenation of alkynes can be done by using Lindlar's catalyst.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




