
Iodine oxidises sodium borohydride to give:
A) ${B_2}{H_6}$
B) Sodium hydride
C) $HI$
D) ${I_3}^{ - 1}$
Answer
233.1k+ views
Hint: Hydrogen borohydride is a minor reducing agent. It does selective reduction and it reduces substances by transfer of hydride ions (${H^ - }$).
Complete step by step solution:
-Sodium borohydride is an organic compound with molecular formula: $NaB{H_4}$. It is a salt consisting of a tetrahedral ($B{H_4}^ - $).
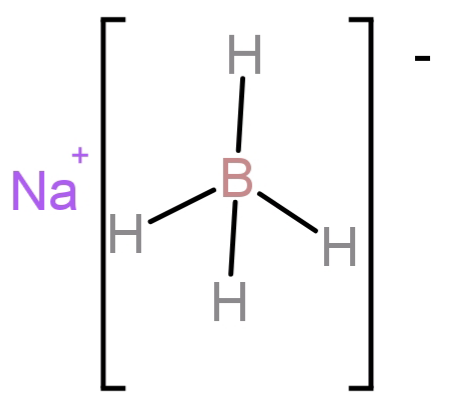
-It is an effective source of nucleophilic hydride ions in polar aprotic solvents like sulfolane, DMSO, DMF, HMPA or diglyme.
- So, it acts as a minor reducing agent and does selective reduction that is it reduces aldehydes and ketones to corresponding alcohols. It does not reduce double bonds. It reduces substance by transfer of the hydride ions.
-When one sodium borohydride molecule reacts with iodine, a borane ($B{H_3}$) molecule is produced along with sodium iodide ($NaI$) and hydrogen iodide ($HI$). Hydrogen iodide then reacts with another sodium borohydride molecule producing another borane and hydrogen iodide molecule. These 2 borane molecules thus do the bridging forming diborane (${B_2}{H_6}$). This is shown in the below reaction:
$NaB{H_4} + {I_2} \to B{H_3} + NaI + HI\xrightarrow{{NaB{H_4}}}B{H_3} + HI$
-This reaction takes place in the presence of Diglyme which acts as a catalyst.
-So, this reaction can also be written as:
$2NaB{H_4} + {I_2}\xrightarrow{{Diglyme}}2NaI + {B_2}{H_6} + {H_2}$
So, the correct option is: A) ${B_2}{H_6}$
Additional information: Diborane is found to be a rocket propellant because it is highly flammable. It undergoes a highly exothermic complete combustion. But it is unable to undergo complete combustion in a rocket engine and thus produces some amount of boron monoxide ($BO$).
Note: Before attempting read the question carefully about iodine oxidising sodium borohydride and not the other way round. And do not get confused between reducing agent (donates electron) and oxidising agent (accepts electron).
Complete step by step solution:
-Sodium borohydride is an organic compound with molecular formula: $NaB{H_4}$. It is a salt consisting of a tetrahedral ($B{H_4}^ - $).

-It is an effective source of nucleophilic hydride ions in polar aprotic solvents like sulfolane, DMSO, DMF, HMPA or diglyme.
- So, it acts as a minor reducing agent and does selective reduction that is it reduces aldehydes and ketones to corresponding alcohols. It does not reduce double bonds. It reduces substance by transfer of the hydride ions.
-When one sodium borohydride molecule reacts with iodine, a borane ($B{H_3}$) molecule is produced along with sodium iodide ($NaI$) and hydrogen iodide ($HI$). Hydrogen iodide then reacts with another sodium borohydride molecule producing another borane and hydrogen iodide molecule. These 2 borane molecules thus do the bridging forming diborane (${B_2}{H_6}$). This is shown in the below reaction:
$NaB{H_4} + {I_2} \to B{H_3} + NaI + HI\xrightarrow{{NaB{H_4}}}B{H_3} + HI$
-This reaction takes place in the presence of Diglyme which acts as a catalyst.
-So, this reaction can also be written as:
$2NaB{H_4} + {I_2}\xrightarrow{{Diglyme}}2NaI + {B_2}{H_6} + {H_2}$
So, the correct option is: A) ${B_2}{H_6}$
Additional information: Diborane is found to be a rocket propellant because it is highly flammable. It undergoes a highly exothermic complete combustion. But it is unable to undergo complete combustion in a rocket engine and thus produces some amount of boron monoxide ($BO$).
Note: Before attempting read the question carefully about iodine oxidising sodium borohydride and not the other way round. And do not get confused between reducing agent (donates electron) and oxidising agent (accepts electron).
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




