
In the Rutherford experiment, \[\alpha - \] particles are scattered from a nucleus as shown. Out of the four paths, which path is not possible?
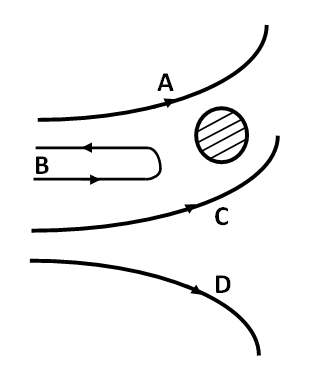
A. D
B. B
C. C
D. A
Answer
232.8k+ views
Hint: To answer this question, one must know Rutherford’s experiment details and the structure of Rutherford’s model of an atom. The dispersion of \[\alpha - \] particles in Rutherford's experiment revealed for the first time that the atom has a nucleus. He noticed that the positive charges in the atom repelled and deflected the positively charged \[\alpha - \] particles. This positively charged area of the atom was given the term "nucleus" by Rutherford.
Complete step by step solution:
We know that like charges repel whereas unlike charges attract. Using this concept along with the knowledge of Rutherford’s model of an atom, we will get the required answer. The structure of alpha particles, also known as alpha rays or alpha radiation, is similar to that of the helium-4 nucleus and is made up of two protons and two neutrons bonded together. They are often formed during the alpha decay process, although they can also be produced by other processes.
The \[\alpha - \] particles carry $ + 2e$ charge on them ( $e$ is the charge of a single electron/proton). Also, the nucleus carries a charge of $ + Ze$ where $Z$ is the atomic number of the atom. Both these particles carry positive charge therefore, the electrostatic force between them is repulsive in nature.
In path D., the \[\alpha - \] particle is going away from the nucleus due to repulsion. Therefore, path D. is possible. In path B., the \[\alpha - \] particle is bouncing back due to repulsion, therefore, this path is also possible.Similarly, in path A., the \[\alpha - \] particle gets repelled by the nucleus and goes away from it. Hence, path A. is also possible.
Whereas in path C. the distance between the \[\alpha - \] particle and the nucleus is decreasing which implies that the \[\alpha - \] particle and the nucleus are attracting each other, which is not true. Therefore, path C. is not possible.
Hence, option C is the answer.
Note: An atom's positive charge and the majority of its mass are concentrated in its tiny nucleus, which serves to resist any incoming positive charges. According to Rutherford's theory, an atom's nucleus is surrounded by electrons that are negatively charged. He also asserted that the electrons that surround the nucleus travel in a circular pattern at extremely high speeds. He gave these elliptical routes the name orbits.
Complete step by step solution:
We know that like charges repel whereas unlike charges attract. Using this concept along with the knowledge of Rutherford’s model of an atom, we will get the required answer. The structure of alpha particles, also known as alpha rays or alpha radiation, is similar to that of the helium-4 nucleus and is made up of two protons and two neutrons bonded together. They are often formed during the alpha decay process, although they can also be produced by other processes.
The \[\alpha - \] particles carry $ + 2e$ charge on them ( $e$ is the charge of a single electron/proton). Also, the nucleus carries a charge of $ + Ze$ where $Z$ is the atomic number of the atom. Both these particles carry positive charge therefore, the electrostatic force between them is repulsive in nature.
In path D., the \[\alpha - \] particle is going away from the nucleus due to repulsion. Therefore, path D. is possible. In path B., the \[\alpha - \] particle is bouncing back due to repulsion, therefore, this path is also possible.Similarly, in path A., the \[\alpha - \] particle gets repelled by the nucleus and goes away from it. Hence, path A. is also possible.
Whereas in path C. the distance between the \[\alpha - \] particle and the nucleus is decreasing which implies that the \[\alpha - \] particle and the nucleus are attracting each other, which is not true. Therefore, path C. is not possible.
Hence, option C is the answer.
Note: An atom's positive charge and the majority of its mass are concentrated in its tiny nucleus, which serves to resist any incoming positive charges. According to Rutherford's theory, an atom's nucleus is surrounded by electrons that are negatively charged. He also asserted that the electrons that surround the nucleus travel in a circular pattern at extremely high speeds. He gave these elliptical routes the name orbits.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Dual Nature of Radiation and Matter Class 12 Physics Chapter 11 CBSE Notes - 2025-26

Understanding the Electric Field of a Uniformly Charged Ring

JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

Derivation of Equation of Trajectory Explained for Students

Understanding Electromagnetic Waves and Their Importance




