
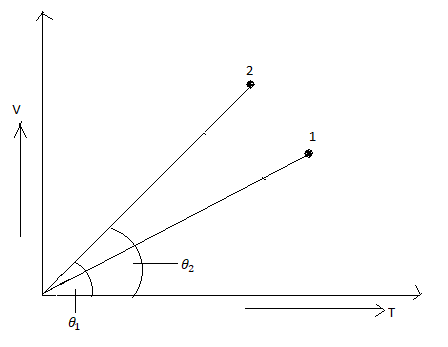
In the following V-T diagram what is the relation between \[{P_1}\] and \[{P_2}\]:
A) ${{\text{P}}_2} = {{\text{P}}_1}$
B) ${{\text{P}}_2} > {{\text{P}}_1}$
C) ${{\text{P}}_2} < {{\text{P}}_1}$
D) Cannot be predicted
Answer
239.4k+ views
Hint:
i)Ideal gas equation: It is the relationship between Boyle’s law, Charles law, and Avogadro’s law.
ii) Boyle’s law: This describes how the pressure of a gas tends to change as the volume of the container.
iii) Charles law: This describes how gas tends to expand when heated.
iv) Avogadro’s law: This describes the relationship between the volumes of gas to the amount of substance of gas present.
Formula used:
Ideal gas equation,${\text{PV = RT}}$,
Here\[\;P = \] pressure of the gas,
\[V = \] volume of the gas,
\[T = \] temperature,
\[R = \] universal gas constant
Complete step by step solution:
It is given that \[{P_1}\]and \[{P_2}\] is the pressure from the graph.
Therefore, the ideal gas equation can be re-written as, $\dfrac{{\text{V}}}{{\text{T}}} = \dfrac{{\text{R}}}{{\text{P}}}$
From the given graph we see that the slope of \[2\,\] is greater than the slope of\[1\].
Therefore, we can write that, $\dfrac{{{{\text{V}}_2}}}{{{{\text{T}}_2}}} > \dfrac{{{{\text{V}}_1}}}{{{{\text{T}}_1}}}$
From the ideal gas equation, $\dfrac{1}{{{{\text{P}}_2}}} > \dfrac{1}{{{{\text{P}}_1}}}$
So we can write it as,
$ \Rightarrow {{\text{P}}_2} < {{\text{P}}_1}$
Hence the correct option is (C).
Note:
i) By using the ideal gas equation, we can measure the temperature accurately irrespective of their masses and other physical quantities.
ii) Boyle’s law in thermodynamics explains the volume of a gas is inversely proportional to its pressure exerted by a gas at a constant temperature.
iii) Charles law in thermodynamics defines where the volume of a fixed mass of a dry gas is directly proportional to its absolute temperature.
iv) Avogadro’s law in thermodynamics where number of atoms/ molecules of a gas is directly proportional to the volume occupied by the gas at a constant temperature.
v) Calorimeter is a device used to measure the heat energy added to the system
i)Ideal gas equation: It is the relationship between Boyle’s law, Charles law, and Avogadro’s law.
ii) Boyle’s law: This describes how the pressure of a gas tends to change as the volume of the container.
iii) Charles law: This describes how gas tends to expand when heated.
iv) Avogadro’s law: This describes the relationship between the volumes of gas to the amount of substance of gas present.
Formula used:
Ideal gas equation,${\text{PV = RT}}$,
Here\[\;P = \] pressure of the gas,
\[V = \] volume of the gas,
\[T = \] temperature,
\[R = \] universal gas constant
Complete step by step solution:
It is given that \[{P_1}\]and \[{P_2}\] is the pressure from the graph.
Therefore, the ideal gas equation can be re-written as, $\dfrac{{\text{V}}}{{\text{T}}} = \dfrac{{\text{R}}}{{\text{P}}}$
From the given graph we see that the slope of \[2\,\] is greater than the slope of\[1\].
Therefore, we can write that, $\dfrac{{{{\text{V}}_2}}}{{{{\text{T}}_2}}} > \dfrac{{{{\text{V}}_1}}}{{{{\text{T}}_1}}}$
From the ideal gas equation, $\dfrac{1}{{{{\text{P}}_2}}} > \dfrac{1}{{{{\text{P}}_1}}}$
So we can write it as,
$ \Rightarrow {{\text{P}}_2} < {{\text{P}}_1}$
Hence the correct option is (C).
Note:
i) By using the ideal gas equation, we can measure the temperature accurately irrespective of their masses and other physical quantities.
ii) Boyle’s law in thermodynamics explains the volume of a gas is inversely proportional to its pressure exerted by a gas at a constant temperature.
iii) Charles law in thermodynamics defines where the volume of a fixed mass of a dry gas is directly proportional to its absolute temperature.
iv) Avogadro’s law in thermodynamics where number of atoms/ molecules of a gas is directly proportional to the volume occupied by the gas at a constant temperature.
v) Calorimeter is a device used to measure the heat energy added to the system
Recently Updated Pages
Dimensions of Charge: Dimensional Formula, Derivation, SI Units & Examples

How to Calculate Moment of Inertia: Step-by-Step Guide & Formulas

Circuit Switching vs Packet Switching: Key Differences Explained

Dimensions of Pressure in Physics: Formula, Derivation & SI Unit

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE General Topics in Chemistry Important Concepts and Tips

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Understanding Electromagnetic Waves and Their Importance

Common Ion Effect: Concept, Applications, and Problem-Solving

Hybridisation in Chemistry – Concept, Types & Applications

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

CBSE Notes Class 11 Physics Chapter 4 - Laws of Motion - 2025-26

CBSE Notes Class 11 Physics Chapter 14 - Waves - 2025-26

CBSE Notes Class 11 Physics Chapter 9 - Mechanical Properties of Fluids - 2025-26

CBSE Notes Class 11 Physics Chapter 11 - Thermodynamics - 2025-26




