
In the above process product \[A\] is
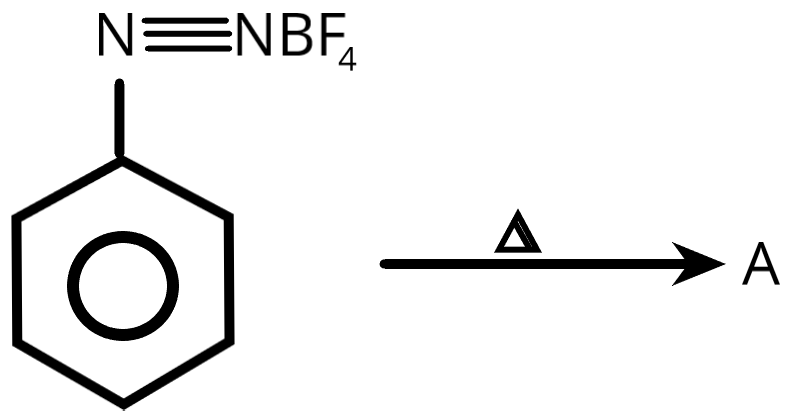
A. Fluorobenzene
B. Benzene
C. \[1,4 - \]difluorobenzene
D. \[1,3 - \]difluorobenzene
Answer
232.8k+ views
Hint: Diazotization reaction is used to prepare multiple organic compounds from aromatic amines. The new group substitutes the diazonium group in the phenyl ring.
Formula Used:
Complete step-by-step answer:In the question, the structure is given by:
Aniline is a prerequisite for the production of fluorobenzene. The procedure known as diazotization is subsequently used to transform the aniline into a diazonium salt. An aromatic amine, such as aniline, is transformed into a diazonium salt during diazotization. The chemical formula of the diazonium salt is\[R{N_2}Cl\], where R represents a benzene ring. After obtaining diazonium salt, we heat it with\[HB{F_4}\] to produce fluorobenzene, the desired product.
First, hydro fluoroboric acid \[(HB{F_4})\] is used to transform benzene diazonium chloride into benzene diazonium fluoroborate, which is then heated to produce fluorobenzene and the byproducts dinitrogen and boron trifluoride.
Schiemann-Balz reaction is the name given to the process of converting benzene diazonium salt into fluorobenzene \[NaB{F_4}\] can also be used in place of \[HB{F_4}\] to create benzene diazonium fluoroborate.
The response is provided below:
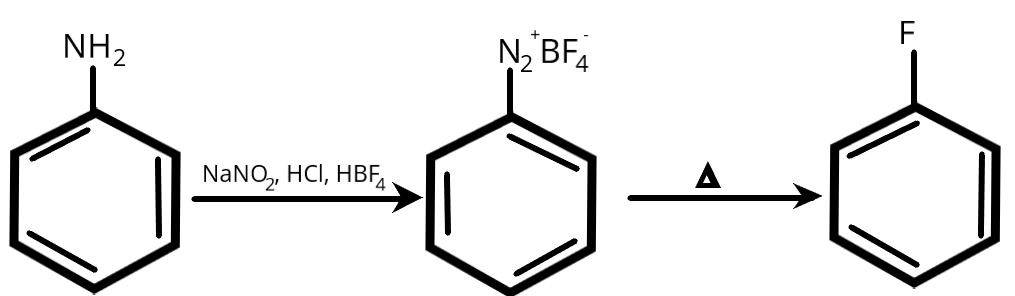
Therefore, the correct name is Fluorobenzene .
Option ‘A’ is correct
Note:We must talk about the structure of fluorobenzene. As a result, the name implies that it is a molecule made of a benzene and a fluorine atom with the chemical formula \[{C_6}{H_5}F\]. The diazotization procedure is a part of the fluorobenzene manufacturing process. It should be noted that Fluorobenzene is a solvent that can be used with extremely reactive substances.
Formula Used:
Complete step-by-step answer:In the question, the structure is given by:

Aniline is a prerequisite for the production of fluorobenzene. The procedure known as diazotization is subsequently used to transform the aniline into a diazonium salt. An aromatic amine, such as aniline, is transformed into a diazonium salt during diazotization. The chemical formula of the diazonium salt is\[R{N_2}Cl\], where R represents a benzene ring. After obtaining diazonium salt, we heat it with\[HB{F_4}\] to produce fluorobenzene, the desired product.
First, hydro fluoroboric acid \[(HB{F_4})\] is used to transform benzene diazonium chloride into benzene diazonium fluoroborate, which is then heated to produce fluorobenzene and the byproducts dinitrogen and boron trifluoride.
Schiemann-Balz reaction is the name given to the process of converting benzene diazonium salt into fluorobenzene \[NaB{F_4}\] can also be used in place of \[HB{F_4}\] to create benzene diazonium fluoroborate.
The response is provided below:

Therefore, the correct name is Fluorobenzene .
Option ‘A’ is correct
Note:We must talk about the structure of fluorobenzene. As a result, the name implies that it is a molecule made of a benzene and a fluorine atom with the chemical formula \[{C_6}{H_5}F\]. The diazotization procedure is a part of the fluorobenzene manufacturing process. It should be noted that Fluorobenzene is a solvent that can be used with extremely reactive substances.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




