
In buna-S, the symbol ‘Bu’ stands for:
(A) 1 – butene
(B) n – butene
(C) 2 – butene
(D) Butadiene
Answer
233.1k+ views
Hint: Buna – S is a random copolymer which is formed by the emulsion polymerization of a mixture of butadiene and styrene in the ratio of \[1:3\]. This reaction takes place in the presence of a peroxide catalyst at \[5^\circ \] C and therefore the product is called cold rubber. The rubber obtained is also called Styrene butadiene rubber (SBR) or commonly known as Buna – S.
Complete Step-by-Step Solution:
Buna – S is a more commonly used name for a polymer named styrene butadiene rubber. The structure of Buna – S is given by:
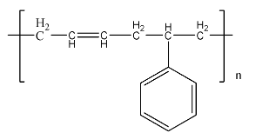
Buna – S is formed using the monomers butadiene and styrene. The reaction of this process can be given as:

In the word Buna – S, the Bu stands for Butadiene, since butadiene is one of the constituent monomers of the given polymer. Also, Na stands for sodium and S represents the monomer Styrene used for the synthesis of the given polymer.
Hence, Option D is the correct option.
Note: Buna -S can be derived from the monomers, butadiene and styrene using two different processes. These processes involve polymer formation from either a solution or an emulsion. The process solution is known as S-SBR while the process which prefers emulsion is known as E-SBR.
Complete Step-by-Step Solution:
Buna – S is a more commonly used name for a polymer named styrene butadiene rubber. The structure of Buna – S is given by:

Buna – S is formed using the monomers butadiene and styrene. The reaction of this process can be given as:

In the word Buna – S, the Bu stands for Butadiene, since butadiene is one of the constituent monomers of the given polymer. Also, Na stands for sodium and S represents the monomer Styrene used for the synthesis of the given polymer.
Hence, Option D is the correct option.
Note: Buna -S can be derived from the monomers, butadiene and styrene using two different processes. These processes involve polymer formation from either a solution or an emulsion. The process solution is known as S-SBR while the process which prefers emulsion is known as E-SBR.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




