
IIdentify the product in following order
$3,4,5 - Tribromoaniline \xrightarrow[\left ( ii \right )H_3PO_4]{\left ( i \right )Diazotisation}$?
A. 3,4,5-Tribromobenzene
B. 1,2,3-Tribromobenzene
C. 2,4,6-Tribromobenzene
D. 3,4,5-Tribromo nitro benzene
E. 3,4,5-Tribromo phenol
Answer
242.1k+ views
Hint: 3,4,5-Tribromoaniline is a compound containing primary aromatic amine with three bromine groups at 3, 4 and 5 positions of aniline. Here in the first step diazotization is performed and then acid is given which will provide its proton in the reaction. Diazotization reaction is a type of coupling reaction for primary amine compounds.
Complete Step by Step Solution:
In the first step, a diazotisation reaction will take place where sodium nitrite and hydrochloric acid at \[{0^ \circ }\]C condition react to give nitrous acid, \[HONO\] which attacks the primary aromatic amine to give diazonium salt i.e., 3,4,5-Tribromobenzene diazonium salt. This then reacts with hypo phosphorus acid reagent which gives its hydrogen to the carbon of benzene ring where diazonium group is attached. So, from the final product nitrogen group is removed and hydrogen is present in its place. The name of the final compound is 1,2,3-tribromobenzene which is option B.
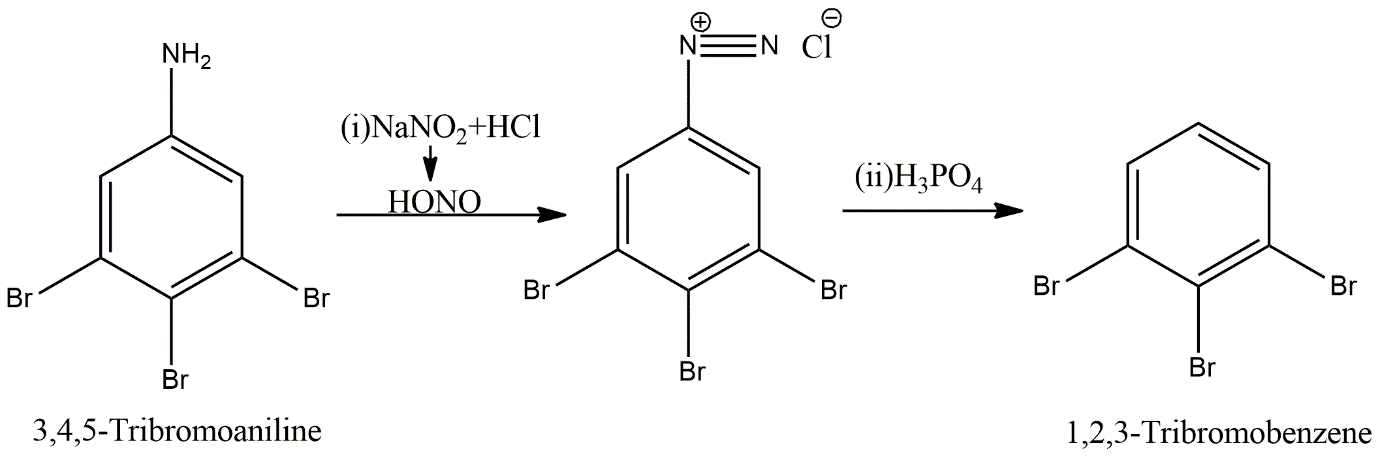
Image: Diazotisation reaction and further treatment with acid of 3,4,5-Tribromoaniline.
So, option B is correct. 2
Note: Ethanimine are the organic compounds having imine functional group i.e.,\[ - C = N - \]. This reacts with water and forms a compound containing a carbonyl functional group such as ketone or aldehyde. In this question acetonitrile is given so it forms acetaldehyde. Here, the overall number of carbons remains the same.
Complete Step by Step Solution:
In the first step, a diazotisation reaction will take place where sodium nitrite and hydrochloric acid at \[{0^ \circ }\]C condition react to give nitrous acid, \[HONO\] which attacks the primary aromatic amine to give diazonium salt i.e., 3,4,5-Tribromobenzene diazonium salt. This then reacts with hypo phosphorus acid reagent which gives its hydrogen to the carbon of benzene ring where diazonium group is attached. So, from the final product nitrogen group is removed and hydrogen is present in its place. The name of the final compound is 1,2,3-tribromobenzene which is option B.

Image: Diazotisation reaction and further treatment with acid of 3,4,5-Tribromoaniline.
So, option B is correct. 2
Note: Ethanimine are the organic compounds having imine functional group i.e.,\[ - C = N - \]. This reacts with water and forms a compound containing a carbonyl functional group such as ketone or aldehyde. In this question acetonitrile is given so it forms acetaldehyde. Here, the overall number of carbons remains the same.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
CBSE Class 12 Chemistry Question Paper 2026 PDF Download (All Sets) with Answer Key

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More




