
IIdentify the product in following order
$3,4,5 - Tribromoaniline \xrightarrow[\left ( ii \right )H_3PO_4]{\left ( i \right )Diazotisation}$?
A. 3,4,5-Tribromobenzene
B. 1,2,3-Tribromobenzene
C. 2,4,6-Tribromobenzene
D. 3,4,5-Tribromo nitro benzene
E. 3,4,5-Tribromo phenol
Answer
232.8k+ views
Hint: 3,4,5-Tribromoaniline is a compound containing primary aromatic amine with three bromine groups at 3, 4 and 5 positions of aniline. Here in the first step diazotization is performed and then acid is given which will provide its proton in the reaction. Diazotization reaction is a type of coupling reaction for primary amine compounds.
Complete Step by Step Solution:
In the first step, a diazotisation reaction will take place where sodium nitrite and hydrochloric acid at \[{0^ \circ }\]C condition react to give nitrous acid, \[HONO\] which attacks the primary aromatic amine to give diazonium salt i.e., 3,4,5-Tribromobenzene diazonium salt. This then reacts with hypo phosphorus acid reagent which gives its hydrogen to the carbon of benzene ring where diazonium group is attached. So, from the final product nitrogen group is removed and hydrogen is present in its place. The name of the final compound is 1,2,3-tribromobenzene which is option B.
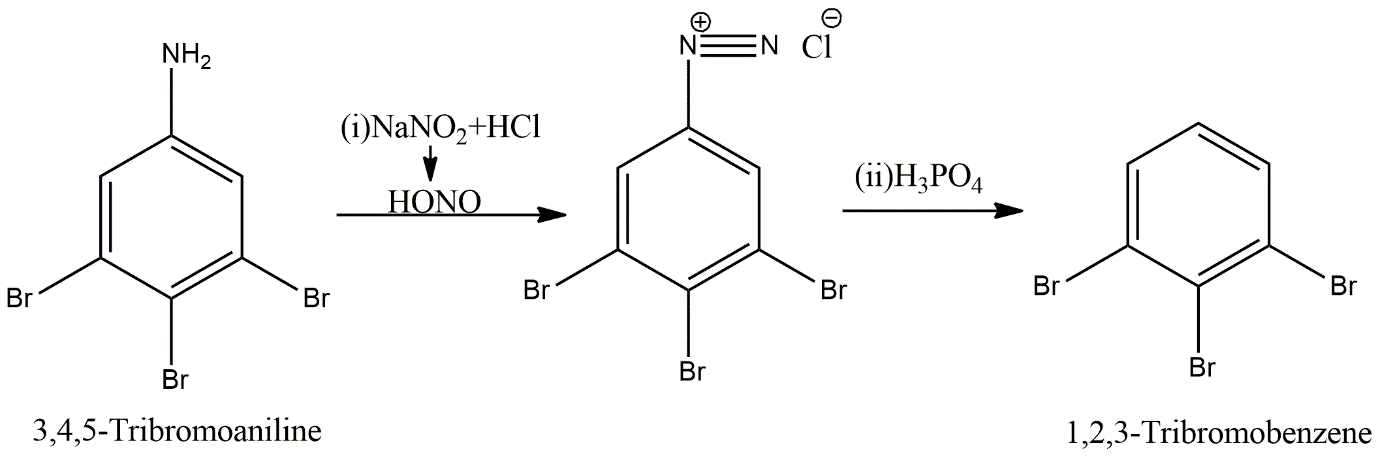
Image: Diazotisation reaction and further treatment with acid of 3,4,5-Tribromoaniline.
So, option B is correct. 2
Note: Ethanimine are the organic compounds having imine functional group i.e.,\[ - C = N - \]. This reacts with water and forms a compound containing a carbonyl functional group such as ketone or aldehyde. In this question acetonitrile is given so it forms acetaldehyde. Here, the overall number of carbons remains the same.
Complete Step by Step Solution:
In the first step, a diazotisation reaction will take place where sodium nitrite and hydrochloric acid at \[{0^ \circ }\]C condition react to give nitrous acid, \[HONO\] which attacks the primary aromatic amine to give diazonium salt i.e., 3,4,5-Tribromobenzene diazonium salt. This then reacts with hypo phosphorus acid reagent which gives its hydrogen to the carbon of benzene ring where diazonium group is attached. So, from the final product nitrogen group is removed and hydrogen is present in its place. The name of the final compound is 1,2,3-tribromobenzene which is option B.

Image: Diazotisation reaction and further treatment with acid of 3,4,5-Tribromoaniline.
So, option B is correct. 2
Note: Ethanimine are the organic compounds having imine functional group i.e.,\[ - C = N - \]. This reacts with water and forms a compound containing a carbonyl functional group such as ketone or aldehyde. In this question acetonitrile is given so it forms acetaldehyde. Here, the overall number of carbons remains the same.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




