
Hydrocarbon (X), C7H12, on reaction with boron hydride followed by treatment with CH3COOH yields (A). On reductive ozonolysis (A) yields a mixture of two aldehydes, (B) and (C). Of these, only (B) can undergo Cannizzaro reaction. (A) exists in two geometrical isomers, (A - 1) and (A - 2), of which (A - 2) is more stable. Give structures of (X), (A), (B), (C), (A -1), and (A - 2) with proper reasoning.
Answer
233.1k+ views
Hint: As it is given B and C are aldehydes and B can undergo a Cannizzaro reaction, so compound B must not have any $\alpha -H$ but Compound C contains $\alpha -H$. Also, compounds B and C are produced from Ozonolysis, hence compound A should be an alkene and it is produced from X by hydroboration reaction.
Complete Step-by-Step Explanation:
In the given question each structure can be identified by given reaction hints. There are different ways to solve this question, but if we proceed to start from the end to the first reaction in the reverse order then we can easily determine all the structures.
As it is given in the question compounds B and C are both aldehydes but only B can undergo the Cannizzaro reaction which means B is an aldehyde compound with $\alpha -H$atoms. Therefore we can write the possible structures B and C according to hydrocarbon ${{C}_{7}}{{H}_{12}}$.
Now on reductive ozonolysis Compound A gives two aldehydes B and C respectively. In the case of the Ozonolysis reaction unsaturated bonds like alkenes, alkynes, or azo compounds are cleaved with ozone, and carbon-carbon multiple bonds have been replaced by a carbonyl group. But it is mentioned in the question that compound A exists in two geometrical isomers, cis and trans-isomer, in which trans isomer is more stable as there is less steric hindrance than cis-isomer, hence stable trans isomer is (A-2). So compound A must be an alkene. Thereby we can draw possible structures below:
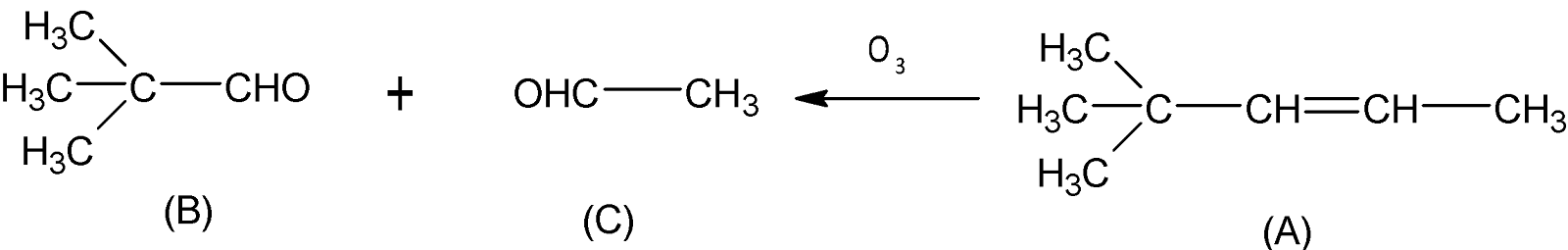
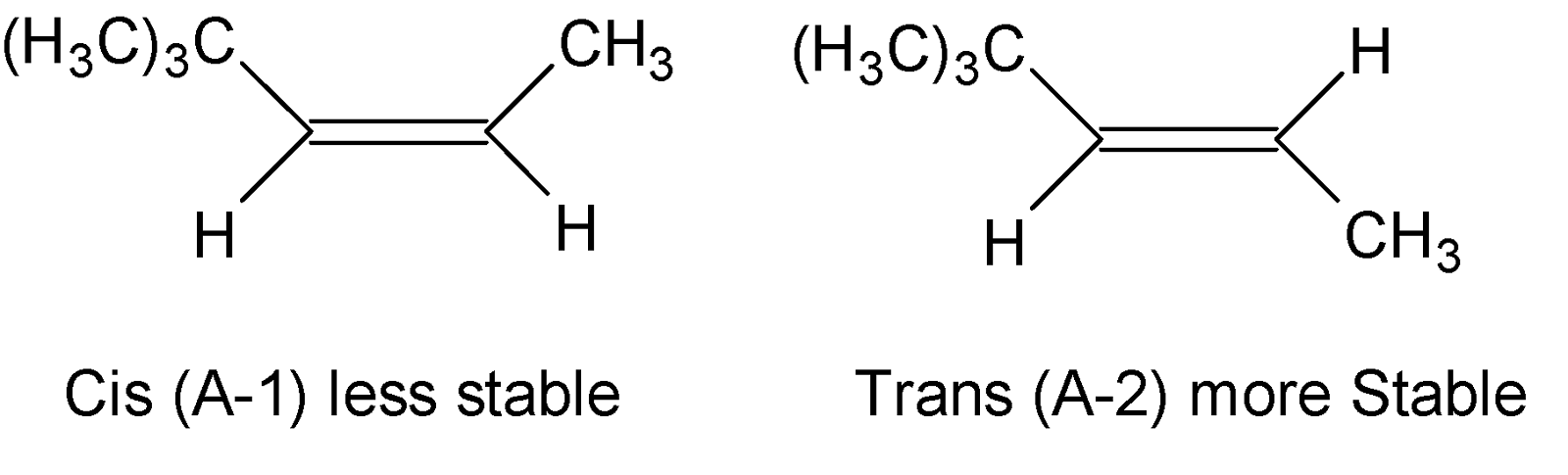
Finally a Hydroboration reaction with $B{{H}_{3}}/C{{H}_{3}}COOH$ , Hydrocarbon X yields compound A. As we know hydroboration reaction is a two-stage reaction that transforms an alkene or alkyne into hydroborane, hence X is an alkyne that can be transformed into an alkene by applying $B{{H}_{3}}/C{{H}_{3}}COOH$( Since compound X cannot be an alkene because then it produces an alkane compound).
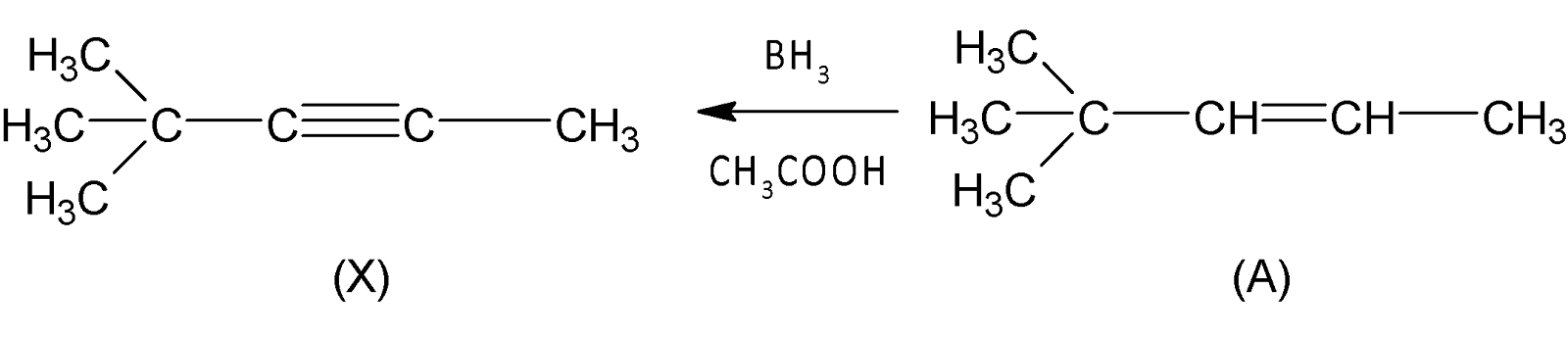
Thus, compound (X) is an alkyne.
Note: To approach this type of question, we should know about these important name reactions such as the Cannizzaro reaction, aldol reaction, Wittig reaction, Reimer-Tiemann reaction, etc and also should familiar with very popular oxidative and reductive reagents in Organic chemistry. Once we are familiar with these reactions and reagents we can easily solve this kind of problem by understanding the given hint.
Complete Step-by-Step Explanation:
In the given question each structure can be identified by given reaction hints. There are different ways to solve this question, but if we proceed to start from the end to the first reaction in the reverse order then we can easily determine all the structures.
As it is given in the question compounds B and C are both aldehydes but only B can undergo the Cannizzaro reaction which means B is an aldehyde compound with $\alpha -H$atoms. Therefore we can write the possible structures B and C according to hydrocarbon ${{C}_{7}}{{H}_{12}}$.
Now on reductive ozonolysis Compound A gives two aldehydes B and C respectively. In the case of the Ozonolysis reaction unsaturated bonds like alkenes, alkynes, or azo compounds are cleaved with ozone, and carbon-carbon multiple bonds have been replaced by a carbonyl group. But it is mentioned in the question that compound A exists in two geometrical isomers, cis and trans-isomer, in which trans isomer is more stable as there is less steric hindrance than cis-isomer, hence stable trans isomer is (A-2). So compound A must be an alkene. Thereby we can draw possible structures below:

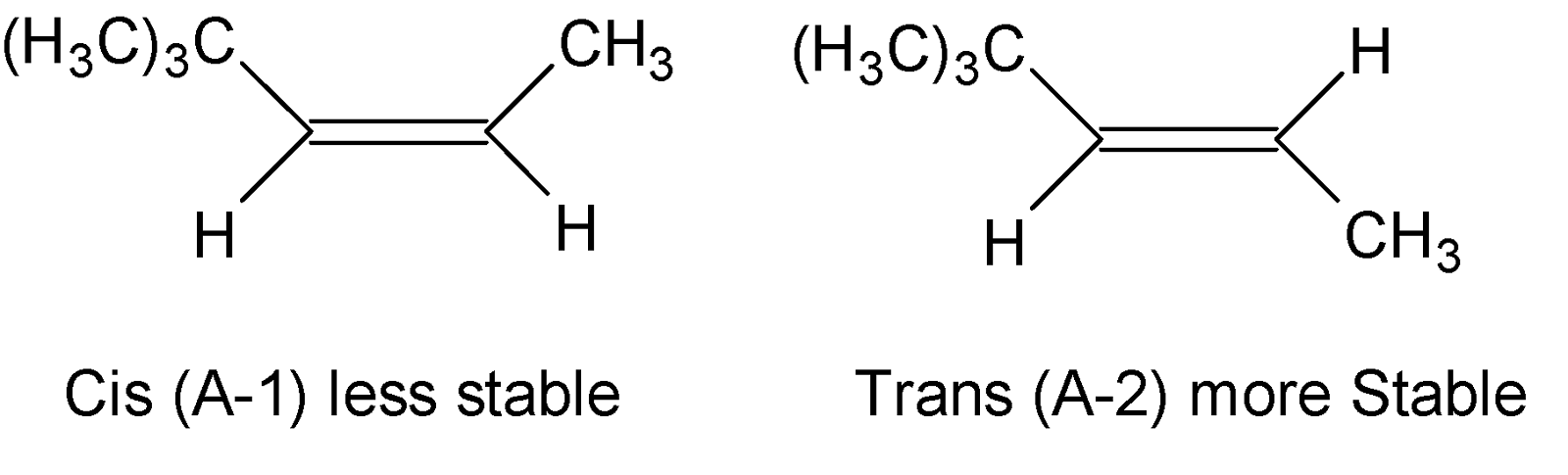
Finally a Hydroboration reaction with $B{{H}_{3}}/C{{H}_{3}}COOH$ , Hydrocarbon X yields compound A. As we know hydroboration reaction is a two-stage reaction that transforms an alkene or alkyne into hydroborane, hence X is an alkyne that can be transformed into an alkene by applying $B{{H}_{3}}/C{{H}_{3}}COOH$( Since compound X cannot be an alkene because then it produces an alkane compound).

Thus, compound (X) is an alkyne.
Note: To approach this type of question, we should know about these important name reactions such as the Cannizzaro reaction, aldol reaction, Wittig reaction, Reimer-Tiemann reaction, etc and also should familiar with very popular oxidative and reductive reagents in Organic chemistry. Once we are familiar with these reactions and reagents we can easily solve this kind of problem by understanding the given hint.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




