
Hofmann's hypobromite reaction affords a method of:
- A. Preparing a tertiary amine
B. Preparing a mixture of amines
C. Stepping down a series
D. Stepping up a series
- A. Preparing a tertiary amine
Answer
233.1k+ views
Hint: Hoffmann’s hypobromite reaction mechanism is used and because of deprotonation reaction affords a mechanism of stepping down a series.
Complete step-by-step solution:Alkali is typically used as a strong base to attack the amide during the Hoffmann bromate reaction, which results in deprotonation and the subsequent formation of an anion. A primary amide can be changed into a primary amine with one less carbon atom using this process. This is done by heating the main amide with a solution of water, a strong base, and a halogen (chlorine or bromine).
Step 1: The strong base's hydroxide ion assaults the amide. Now that the amide has been deprotonated, water and the amide anion have been created.
Step 2: An alpha substitution reaction now takes place as the anion attacks the diatomic bromine. N-Bromamide and Br- anion are created as a result of the bromine-bromine bond breaking.
Step 3: The base now attacks the N-Bromamide once more, which causes its deprotonation and the production of water as well as the bromamide anion.
Step 4: This bromamide anion undergoes rearrangement so that the previously bonded ethyl group (or any other R- group) with the carbonyl carbon now binds with the nitrogen. The bromide anion that forms simultaneously departs from the complex. An isocyanate is produced as a result.
Step 5: A nucleophilic addition reaction causes carbamic acid to develop when water is added to the isocyanate.
step 6 : The carbamic acid now releases carbon dioxide, resulting in a negatively charged nitrogen linked to one hydrogen and the ethyl group in step 6. The necessary primary amine is created when this is protonated by the water.
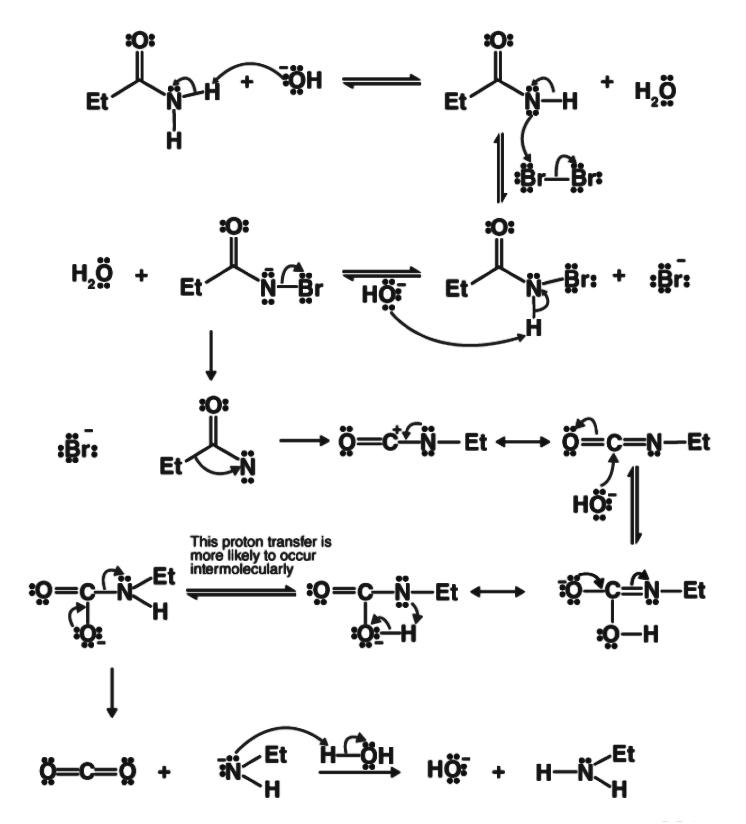
Hofmann's hypobromite reaction affords a method of stepping down a series.
\[C{{H}_{3}}CON{{H}_{2}}+B{{r}_{2}}+4KOH\to C{{H}_{3}}N{{H}_{2}}+{{K}_{2}}C{{O}_{3}}+2KBr+2{{H}_{2}}O\]
Hence, the correct option is option C which is stepping down a series.
Note: While writing the mechanism of the reaction we should always keep in mind the number of free radicals and the movement of the radicals.
Complete step-by-step solution:Alkali is typically used as a strong base to attack the amide during the Hoffmann bromate reaction, which results in deprotonation and the subsequent formation of an anion. A primary amide can be changed into a primary amine with one less carbon atom using this process. This is done by heating the main amide with a solution of water, a strong base, and a halogen (chlorine or bromine).
Step 1: The strong base's hydroxide ion assaults the amide. Now that the amide has been deprotonated, water and the amide anion have been created.
Step 2: An alpha substitution reaction now takes place as the anion attacks the diatomic bromine. N-Bromamide and Br- anion are created as a result of the bromine-bromine bond breaking.
Step 3: The base now attacks the N-Bromamide once more, which causes its deprotonation and the production of water as well as the bromamide anion.
Step 4: This bromamide anion undergoes rearrangement so that the previously bonded ethyl group (or any other R- group) with the carbonyl carbon now binds with the nitrogen. The bromide anion that forms simultaneously departs from the complex. An isocyanate is produced as a result.
Step 5: A nucleophilic addition reaction causes carbamic acid to develop when water is added to the isocyanate.
step 6 : The carbamic acid now releases carbon dioxide, resulting in a negatively charged nitrogen linked to one hydrogen and the ethyl group in step 6. The necessary primary amine is created when this is protonated by the water.

Hofmann's hypobromite reaction affords a method of stepping down a series.
\[C{{H}_{3}}CON{{H}_{2}}+B{{r}_{2}}+4KOH\to C{{H}_{3}}N{{H}_{2}}+{{K}_{2}}C{{O}_{3}}+2KBr+2{{H}_{2}}O\]
Hence, the correct option is option C which is stepping down a series.
Note: While writing the mechanism of the reaction we should always keep in mind the number of free radicals and the movement of the radicals.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




