
Formamide is
A.$HCON{{H}_{2}}$
B.$C{{H}_{3}}CON{{H}_{2}}$
C.$HCOON{{H}_{4}}$
D.$(HCHO+N{{H}_{3}})$
Answer
232.8k+ views
Hint: Formamide is the simplest monocarboxylic acid amide. It can be synthesized by formal condensation of formic acid and ammonia and it is functionally related to formic acid. To approach this problem we will have to the general structure of the carboxylic acid amide compound.
Complete answer:Generally, amides are derived from carboxylic acids in which the hydroxyl part $(-OH)$is replaced by $-N{{H}_{2}}$ group. For example, a general carboxylic acid, $RCOOH$reacts with ammonia, $N{{H}_{3}}$to form an amide-containing $-CON{{H}_{2}}$group.
$RCOOH+N{{H}_{3}}\to RCON{{H}_{2}}+{{H}_{2}}O$
The structure of amide is shown below:
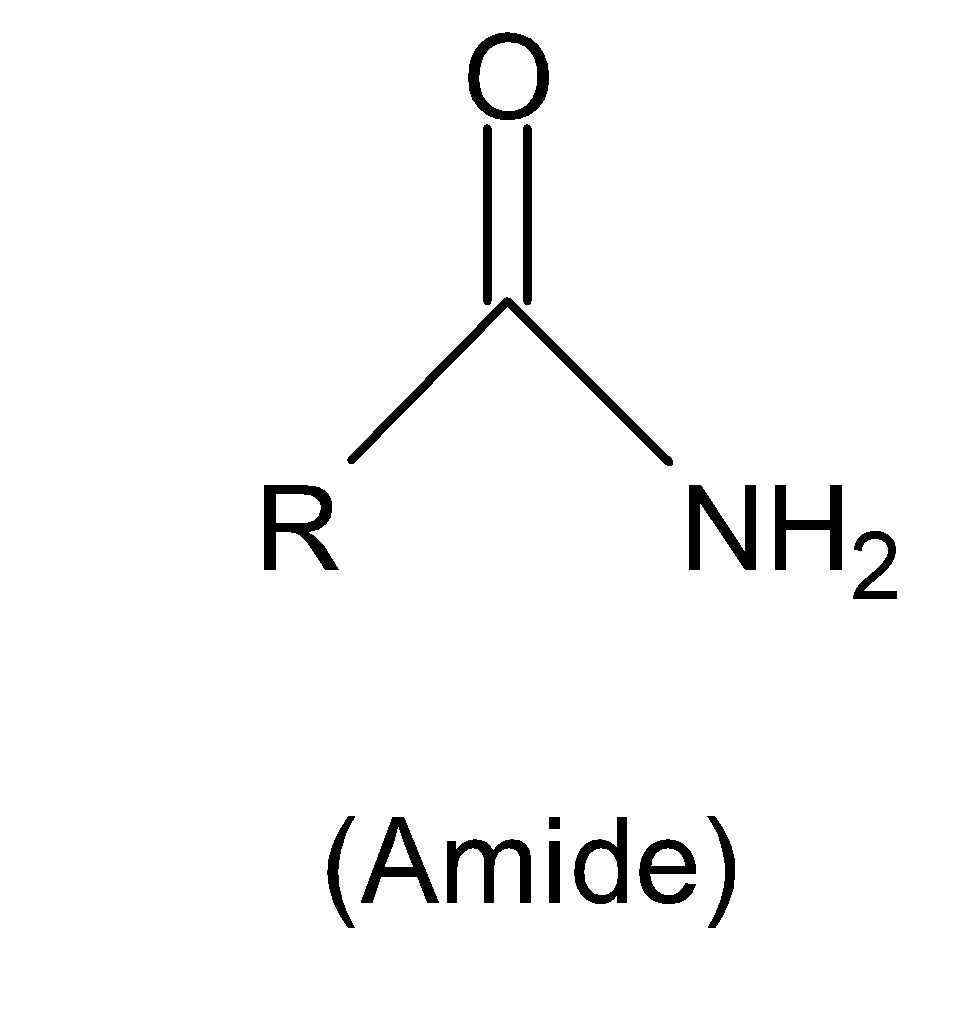
The naming of amide is derived from the carboxylic acid by replacing the ‘’oic acid’’ ending with the word ‘’amide’’.
For example, the chemical formula of formic acid is $HCOOH$and the amide corresponding to formic acid is methanamide,$HCON{{H}_{2}}$.Similarly methanoic acid $C{{H}_{3}}COOH$and its corresponding amide are ethanamide, $C{{H}_{3}}CON{{H}_{2}}$.
As we can see over here that $-CON{{H}_{2}}$groups are present in (A) and (B), so both are compounds of amide in which (A) $HCON{{H}_{2}}$is methanamide (one carbon atom is present) or formamide and (B) $C{{H}_{3}}CON{{H}_{2}}$is ethanamide( two carbon atoms are present).

In (C) $HCOON{{H}_{4}}$is not an amide but an ammonium salt of formic acid.
When formaldehyde, $HCHO$reacts with ammonia, $N{{H}_{3}}$it forms hexamethylenetetramine, ${{(C{{H}_{2}})}_{6}}{{N}_{4}}$ popularly known as urotropine. Hence it is also not an amide compound.
$6HCHO+4N{{H}_{3}}\to {{(C{{H}_{2}})}_{6}}{{N}_{4}}+6{{H}_{2}}O$
Therefore the correct chemical formula of formamide is $HCON{{H}_{2}}$.
Thus, option (A) is correct.
Note: Formamide has many industrial chemical applications in the field of the pharmaceutical industry. It can also be used in the production of papers and can dissolve compounds and form ions, therefore it can be used as a solvent. This is a very toxic chemical to cause birth defects, so women should avoid this chemical in the early stages of pregnancy.
Complete answer:Generally, amides are derived from carboxylic acids in which the hydroxyl part $(-OH)$is replaced by $-N{{H}_{2}}$ group. For example, a general carboxylic acid, $RCOOH$reacts with ammonia, $N{{H}_{3}}$to form an amide-containing $-CON{{H}_{2}}$group.
$RCOOH+N{{H}_{3}}\to RCON{{H}_{2}}+{{H}_{2}}O$
The structure of amide is shown below:

The naming of amide is derived from the carboxylic acid by replacing the ‘’oic acid’’ ending with the word ‘’amide’’.
For example, the chemical formula of formic acid is $HCOOH$and the amide corresponding to formic acid is methanamide,$HCON{{H}_{2}}$.Similarly methanoic acid $C{{H}_{3}}COOH$and its corresponding amide are ethanamide, $C{{H}_{3}}CON{{H}_{2}}$.
As we can see over here that $-CON{{H}_{2}}$groups are present in (A) and (B), so both are compounds of amide in which (A) $HCON{{H}_{2}}$is methanamide (one carbon atom is present) or formamide and (B) $C{{H}_{3}}CON{{H}_{2}}$is ethanamide( two carbon atoms are present).

In (C) $HCOON{{H}_{4}}$is not an amide but an ammonium salt of formic acid.
When formaldehyde, $HCHO$reacts with ammonia, $N{{H}_{3}}$it forms hexamethylenetetramine, ${{(C{{H}_{2}})}_{6}}{{N}_{4}}$ popularly known as urotropine. Hence it is also not an amide compound.
$6HCHO+4N{{H}_{3}}\to {{(C{{H}_{2}})}_{6}}{{N}_{4}}+6{{H}_{2}}O$
Therefore the correct chemical formula of formamide is $HCON{{H}_{2}}$.
Thus, option (A) is correct.
Note: Formamide has many industrial chemical applications in the field of the pharmaceutical industry. It can also be used in the production of papers and can dissolve compounds and form ions, therefore it can be used as a solvent. This is a very toxic chemical to cause birth defects, so women should avoid this chemical in the early stages of pregnancy.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




