
Experiment shows that \[{{\rm{H}}_{\rm{2}}}{\rm{O}}\] has a dipole moment while \[{\rm{C}}{{\rm{O}}_{\rm{2}}}\] has not. Point out the structures which best illustrate these facts
A)
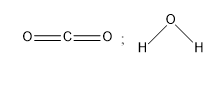
B)
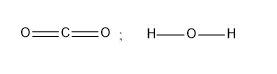
C)
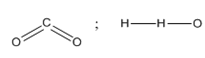
D)
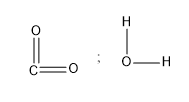
Answer
232.8k+ views
Hint: To find out the hybridization of the Ag atom in the given complex, first we have to calculate the oxidation state of the silver (Ag) atom. Then, we have to find its hybridization using the valence bond theory.
Complete Step by Step Answer:
The diagram of the \[{\rm{C}}{{\rm{O}}_{\rm{2}}}\] molecule is,
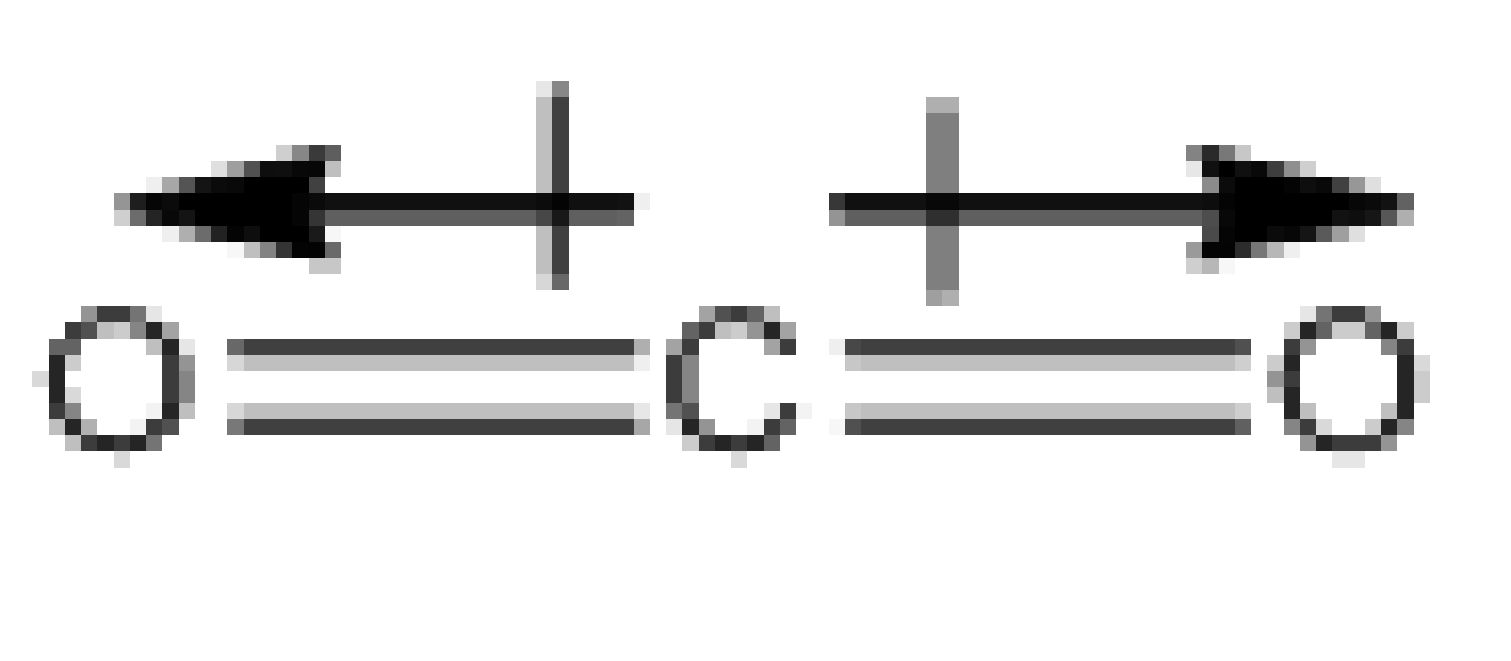
Image: \[{\rm{C}}{{\rm{O}}_{\rm{2}}}\] molecule
So, we see that the shape of the molecule is linear, having the oxygen atom on either end of the carbon atom. The nature of the C=O is polar because of the more electronegative oxygen than carbon. The polarity of the two C=O bonds point exactly opposite to one another, so the individual bond dipoles get cancelled. Therefore, the carbon dioxide molecule has a dipole moment of zero.
The water molecule is as follows:
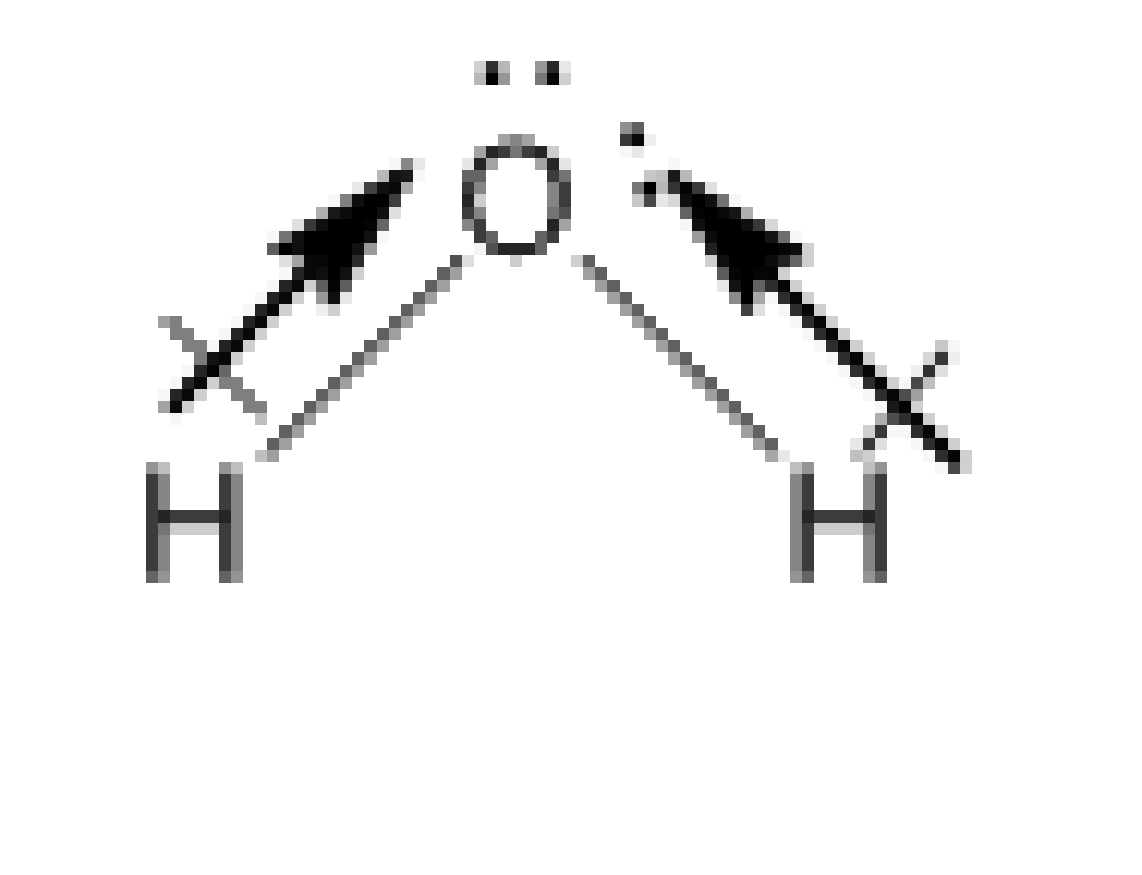
Image: Water molecule
In the water molecule, the two polar bonds of O-H are present. And they point in the same direction which is towards the central atom O. Therefore, the dipole moments of the two bonds are not getting cancelled but added. So, the water molecule has some dipole moment.
Therefore, option A depicts the correct diagram of water and carbon dioxide, that is the linear structure of \[{\rm{C}}{{\rm{O}}_{\rm{2}}}\] and bent structure of water.
Note: The dipole moment's magnitude helps us to identify the nature of the polar bond. The increase of the dipole moment's magnitude tells us the bond's polar nature. Molecules possessing zero dipole moment are non-polar molecules.
Complete Step by Step Answer:
The diagram of the \[{\rm{C}}{{\rm{O}}_{\rm{2}}}\] molecule is,
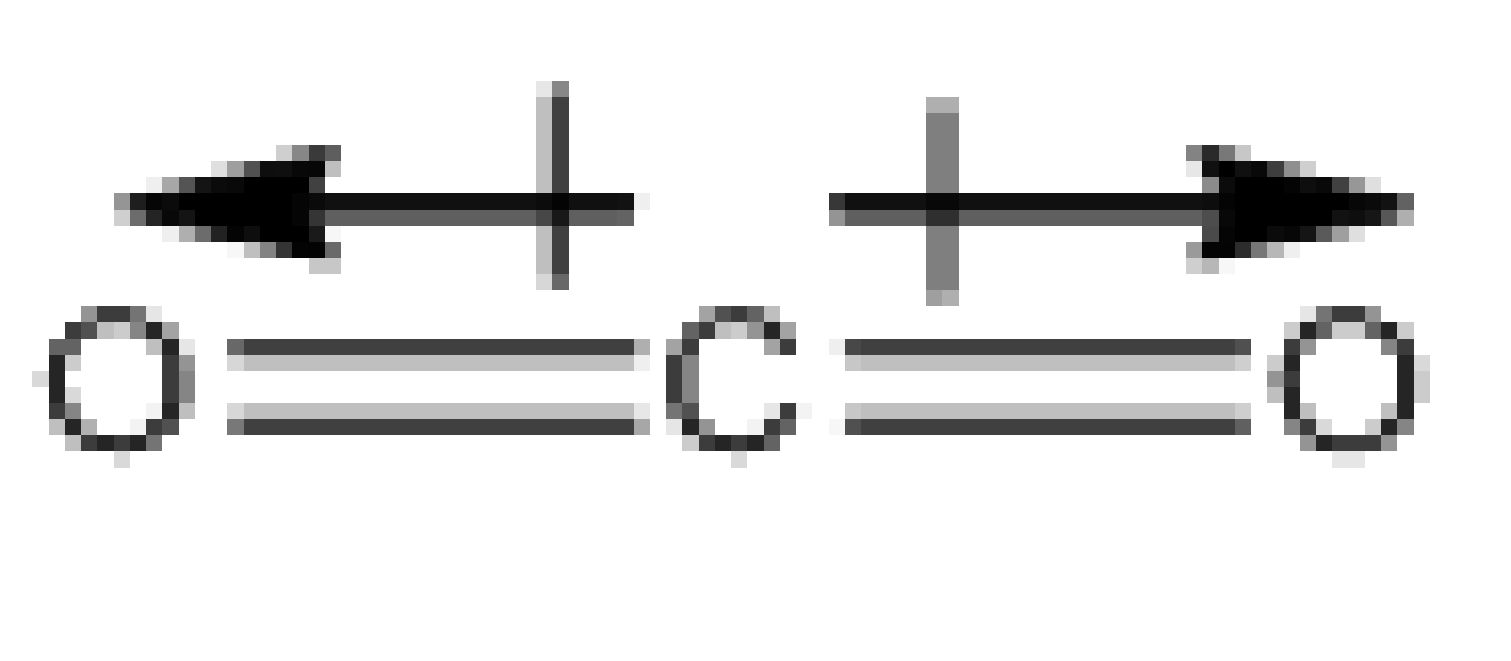
Image: \[{\rm{C}}{{\rm{O}}_{\rm{2}}}\] molecule
So, we see that the shape of the molecule is linear, having the oxygen atom on either end of the carbon atom. The nature of the C=O is polar because of the more electronegative oxygen than carbon. The polarity of the two C=O bonds point exactly opposite to one another, so the individual bond dipoles get cancelled. Therefore, the carbon dioxide molecule has a dipole moment of zero.
The water molecule is as follows:

Image: Water molecule
In the water molecule, the two polar bonds of O-H are present. And they point in the same direction which is towards the central atom O. Therefore, the dipole moments of the two bonds are not getting cancelled but added. So, the water molecule has some dipole moment.
Therefore, option A depicts the correct diagram of water and carbon dioxide, that is the linear structure of \[{\rm{C}}{{\rm{O}}_{\rm{2}}}\] and bent structure of water.
Note: The dipole moment's magnitude helps us to identify the nature of the polar bond. The increase of the dipole moment's magnitude tells us the bond's polar nature. Molecules possessing zero dipole moment are non-polar molecules.
Recently Updated Pages
Area of an Octagon Formula Explained Simply

Absolute Pressure Formula Explained: Key Equation & Examples

Central Angle of a Circle Formula Explained Quickly

Difference Between Vapor and Gas: JEE Main 2026

Difference Between Atom and Molecule: JEE Main 2026

Carbon Dioxide Formula - Definition, Uses and FAQs

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Jan 21 Shift 1 Question Papers with Solutions & Answer Keys – Detailed Day 1 Analysis

JEE Main Response Sheet 2026 Released – Key Dates and Official Updates by NTA

JEE Main 2026 Answer Key OUT – Download Session 1 PDF, Response Sheet & Challenge Link

JEE Main Marks vs Percentile 2026: Calculate Percentile and Rank Using Marks

JEE Main 2026 Jan 22 Shift 1 Today Paper Live Analysis With Detailed Solutions

Other Pages
Happy New Year Wishes 2026 – 100+ Messages, Quotes, Shayari, Images & Status in All Languages

One Day International Cricket

Valentine Week 2026: Complete List of Valentine Week Days & Meaning of Each Day

List of Highest T20 Scores in International Cricket

Makar Sankranti Wishes: Happy Makar Sankranti Wishes in Marathi, Hindi, Kannada, and English

What is the Full Form of UGC? Detailed Guide for Students




