
Compounds containing both amino and COOH groups are known as
A. Diamines
B. unknown
C. Amino acids
D. Enzymes
Answer
233.1k+ views
Hint: The amino group is a functional group which is present in an amine. COOH is the functional group which is present in a carboxylic acid compound.
Complete Step by Step Solution:
Functional groups are the groups that present chemical compounds which show their different chemical properties. Different functional groups show different properties.
Amino acids are organic compounds which combine together to form a protein. Therefore, amino acids are referred to as the building block of proteins.
An amino group is a functional group with the general formula \[R - N{H_2}\]where R is the alkyl group.
The Carboxyl group is a functional group with the general formula R-COOH where R is the alkyl group.
Amino acid is an organic molecule which contains both the amino functional group and carboxyl functional group attached to the central carbon atom. The two bonds of carbon are attached to an alkyl group (R) and a hydrogen atom.
The general structure of amino acids is given below.
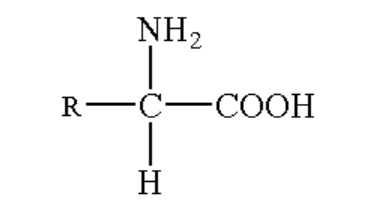
Image: General formula of amino acid.
Thus, the compounds containing both amino and COOH groups are known as amino acids.
Therefore, option C is correct.
Note: There are different amino acids which differ from each other on the basis of the alkyl group attached. There is a total of 20 amino acids which occur naturally among which there are a total of 9 essential amino acids i.e, histidine, leucine, lycine, isoleucine, methionine, valine, phenylalanine, threonine, and tryptophan.
Complete Step by Step Solution:
Functional groups are the groups that present chemical compounds which show their different chemical properties. Different functional groups show different properties.
Amino acids are organic compounds which combine together to form a protein. Therefore, amino acids are referred to as the building block of proteins.
An amino group is a functional group with the general formula \[R - N{H_2}\]where R is the alkyl group.
The Carboxyl group is a functional group with the general formula R-COOH where R is the alkyl group.
Amino acid is an organic molecule which contains both the amino functional group and carboxyl functional group attached to the central carbon atom. The two bonds of carbon are attached to an alkyl group (R) and a hydrogen atom.
The general structure of amino acids is given below.

Image: General formula of amino acid.
Thus, the compounds containing both amino and COOH groups are known as amino acids.
Therefore, option C is correct.
Note: There are different amino acids which differ from each other on the basis of the alkyl group attached. There is a total of 20 amino acids which occur naturally among which there are a total of 9 essential amino acids i.e, histidine, leucine, lycine, isoleucine, methionine, valine, phenylalanine, threonine, and tryptophan.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




