




How to Identify and Classify Types of Redox Reactions with Examples
The types of redox reactions represent a key chapter in JEE Main Chemistry, exploring diverse ways in which oxidation and reduction processes shape chemical change. In daily life, these reactions drive phenomena from metal corrosion and cellular respiration to the extraction of metals and synthesis of key compounds. Understanding how to differentiate, identify, and memorise each class of redox reaction underpins both conceptual clarity and exam-solving speed.
Types of Redox Reactions
All redox reactions involve transfer of electrons, but the patterns differ. JEE and NCERT syllabi classify them mainly as combination, decomposition, displacement (single replacement), disproportionation, and redox titrations. Each type follows set rules of oxidation number change and atom rearrangement in the reaction equation.
- Combination reactions: Two or more substances form a single product with a change in oxidation state.
- Decomposition reactions: A single compound breaks down into simpler products, causing both oxidation and reduction.
- Displacement reactions: An atom/ion replaces another, commonly found as metal and non-metal displacement subtypes.
- Disproportionation reactions: The same element in one substance is both oxidised and reduced.
- Redox titrations: Analytical method where one reactant’s volume needed to completely react with another is measured; involves specific redox couples.
The core principles of redox reactions rely on electron transfer illustrated by these types. For JEE, focus on change in oxidation number and electron movement to quickly classify reaction type.
Each Type with Equations and Examples
The table below summarises the main types of redox reactions, their representative equations, and real-world or exam-centric examples relevant for JEE aspirants.
| Type | General Equation | JEE/Real-life Example | Brief Explanation |
|---|---|---|---|
| Combination | A + B → AB | 2H2 + O2 → 2H2O | Both H and O undergo oxidation state change. Water forms by combining elements. |
| Decomposition | AB → A + B | 2H2O2 → 2H2O + O2 | Single reactant yields two/more products with change in oxidation numbers. |
| Displacement | A + BC → AC + B | Zn + CuSO4 → ZnSO4 + Cu | A more reactive element replaces another; classic in metallurgy. |
| Disproportionation | 2A → A+ + A- | Cl2 + 2OH- → ClO- + Cl- + H2O | Same species acts as oxidant and reductant; both oxidation and reduction resemble. |
| Redox Titration | Ox + Red → Red' + Ox' | KMnO4 titration of FeSO4 solution | Analytical quantification via electron transfer between titrant and analyte. |
Organic redox reactions have similar patterns; for example, alcohol oxidation to aldehydes and reduction of nitro compounds to amines. Always confirm which atom’s oxidation number changes to spot the redox event in organic cases.
How to Identify a Redox Reaction Fast
In MCQs, time-efficient identification of a redox reaction is essential. Use this stepwise hack:
- Note reactants and products, writing their oxidation numbers per atom (use periodic table rules).
- Check if any element’s oxidation number increases (oxidation).
- Check if any element’s oxidation number decreases (reduction).
- If both occur, it is a redox reaction; match electron loss and gain.
- Map the type: combination (forming one), decomposition (splitting), displacement, or disproportionation (single species both changes).
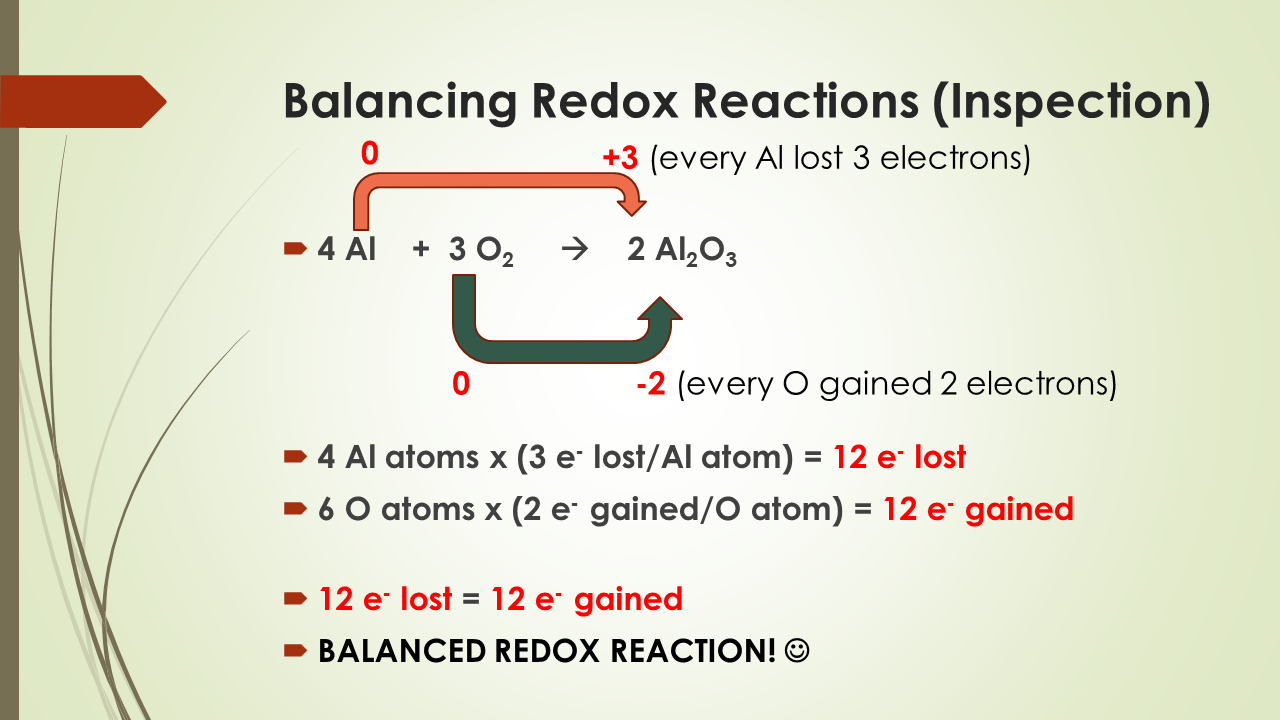
For example, in Zn + 2HCl → ZnCl2 + H2, Zn goes 0 → +2 (oxidation), H goes +1 → 0 (reduction): displacement and redox both. Redox reactions can overlap with other categories, so always check oxidation number change instead of just reactant/product form.
Applications and Importance of Redox Reactions
The practical importance of different types of redox reactions is vast in chemistry and industry:
- Electrochemical cells and energy production depend on redox reactions for batteries, fuel cells, and electrolysis.
- Extraction of metals from ores uses displacement and reduction reactions.
- Everyday processes: respiration, combustion, bleaching, photosynthesis use different redox reaction types.
- Electrochemistry principles and redox titrations are vital in analytical labs and measuring vitamin C content, water quality, etc.
- Corrosion and rusting (iron to Fe2O3) are classic large-scale natural redox reactions.
Understanding the classification of redox reactions helps with mechanisms, balancing by oxidation number and half-reaction methods, and quick recognition in exam problems. The redox reaction into two half processes can simplify even complex equations in competitive exams.
Quick-Reference Table: Types of Redox Reactions
For fast revision, use this summary to compare all main types for JEE Main:
| Redox Type | Key Feature | Classic Example |
|---|---|---|
| Combination | Two combine, both change oxidation state | C + O2 → CO2 |
| Decomposition | One splits; both OX/RD on products | 2KClO3 → 2KCl + 3O2 |
| Displacement | Element replaces another in compound | Fe + CuSO4 → FeSO4 + Cu |
| Disproportionation | One element is oxidised and reduced | 2H2O2 → 2H2O + O2 |
| Redox Titration | Quantifies unknown via electron transfer | Fe2+ vs KMnO4 titration |
For more classification, tricky JEE practice and examples, refer to the official Vedantu guide on types of redox reactions. Always clarify the role of each atom and map oxidation state trends with practice.
Practice and Next Steps
Strengthen your grip by solving problems from PyQ and mock tests: identify both the type and the atoms oxidised/reduced, using the steps above. Tackle special questions with organic redox changes, and remember not all decomposition or combination reactions are redox—check if oxidation numbers actually shift. For deeper insight, explore resources like Vedantu’s redox practice paper and JEE electrochemistry modules.
Learning the types of redox reactions with multiple examples, strategic tricks, and real-life context gives you a major advantage in Chemistry for JEE Main. Always prioritise understanding electron movement and oxidation number changes—they unlock accurate reaction classification and faster MCQ solving, which are key to top exam scores and a strong Chemistry foundation.
FAQs on Types of Redox Reactions: Explanation, Examples, and Exam Tips
1. What are the 5 redox reactions?
The 5 main types of redox reactions are:
- Combination Reactions
- Decomposition Reactions
- Displacement (Single Replacement) Reactions
- Disproportionation Reactions
- Redox Titrations
Each type involves transfer of electrons and changes in oxidation states, making them crucial for JEE, NEET, and board exams.
2. What are the two types of redox?
The two main types of redox processes are:
- Oxidation (loss of electrons or increase in oxidation state)
- Reduction (gain of electrons or decrease in oxidation state)
In every redox reaction, oxidation and reduction occur simultaneously between reactants.
3. What are the most common redox reactions?
The most common redox reactions found in syllabus and real life include:
- Rusting of iron (Fe + O₂ + H₂O → Fe₂O₃)
- Combustion of fuels (CH₄ + 2O₂ → CO₂ + 2H₂O)
- Photosynthesis (6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂)
- Metals reacting with acids (Zn + 2HCl → ZnCl₂ + H₂)
These reactions are important for exam preparation and understanding real-world chemistry.
4. What are the main types of redox reactions?
The main types of redox reactions are:
- Combination (e.g., formation of water from H₂ and O₂)
- Decomposition (e.g., electrolysis of water)
- Displacement (e.g., CuSO₄ + Zn → ZnSO₄ + Cu)
- Disproportionation (e.g., 2H₂O₂ → 2H₂O + O₂)
- Redox Titrations (used in volumetric analysis)
Knowing these helps in classifying any reaction in exams quickly.
5. How many types of redox reactions are there in chemistry?
There are four major types of redox reactions commonly discussed:
1. Combination
2. Decomposition
3. Displacement
4. Disproportionation
Additionally, redox titrations are considered a practical subtype for advanced study.
6. Can you give examples of each type of redox reaction?
Yes, each type of redox reaction has classic examples:
- Combination: H₂ + Cl₂ → 2HCl
- Decomposition: 2H₂O → 2H₂ + O₂
- Displacement: Zn + CuSO₄ → ZnSO₄ + Cu
- Disproportionation: 2H₂O₂ → 2H₂O + O₂
- Redox Titration: Fe²⁺ titrated with KMnO₄
These examples are syllabus-focused and can be used in answers for exams.
7. What is the difference between oxidation and reduction?
Oxidation is the loss of electrons or an increase in oxidation number; reduction is the gain of electrons or a decrease in oxidation number.
- During a redox reaction, one element is oxidized and another is reduced. Learn to track electron flow or changes in oxidation state to answer exam questions efficiently.
8. What type of reaction is a displacement reaction?
Displacement reactions (also called single replacement reactions) are a type of redox reaction where one element replaces another in a compound.
Example:
- Zn + CuSO₄ → ZnSO₄ + Cu
Here, Zn is oxidized and Cu²⁺ is reduced.
9. How to identify a redox reaction in a chemical equation?
To identify a redox reaction:
- Assign oxidation numbers to all atoms.
- Look for changes in oxidation states from reactants to products.
- If at least one element is oxidized and another is reduced, the reaction is redox.
Practice quickly spotting these changes for MCQs and numerical problems.
10. What are the applications of redox reactions in real life?
Redox reactions have many real-world applications:
- Cellular respiration and photosynthesis
- Electrochemical cells (batteries)
- Metallurgy (extraction of metals)
- Bleaching and disinfecting
- Corrosion and rusting
These applications make redox reactions important both in industry and daily life.























