




Introduction
What is a nucleophilic substitution reaction? The term "nucleophilic substitution reaction" refers to a type of organic reaction in which one nucleophile replaces another. It is very similar to the typical displacement reactions seen in chemistry, in which a more reactive element replaces a less reactive element in its salt solution. The group that accepts the electron pairs and is displaced from the carbon is referred to as the "leaving group," and the molecule on which substitution occurs is referred to as the "substrate." The leaving group is either a neutral molecule or an anion.
Nucleophilic Substitution Reaction
In haloalkanes (alkyl halides), the halogen atom is more electronegative than the carbon atom attached to it. As a result, the carbon atom becomes partially positive and the halogen atom becomes partially negative.
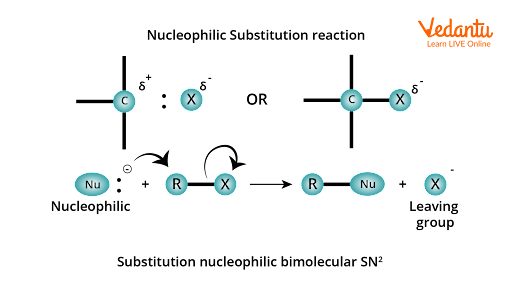
Nucleophilic substitution reaction; a nucleophilic attack on R-X
The presence of this small positive charge on the carbon atom makes it vulnerable to attack by nucleophilic reagents (i.e., reagents possessing a negative charge or a lone pair of electrons). As a result, when a nucleophile stronger than the halide ion approaches the positively charged carbon atom of an alkyl halide, the halogen atom and its bonding pair of electrons are displaced, and a new bond is formed between the carbon atom and the incoming nucleophile.
Nucleophilic substitution reactions occur when a stronger nucleophile displaces a weaker nucleophile, and the atom or group (halide ion in this case) that departs with its bonding pair of electrons is known as the leaving group. The simpler the nucleophilic substitution reaction, the better the leaving group.
Among the halide ions, the leaving groups leave in the following order: I>Br> Cl>F. Due to this, the order of reactivity of haloalkanes is as follows:
I>Br> Cl>F.
Mechanism of Nucleophilic Substitution Reaction
Types of nucleophilic substitution reactions:
SN2 ( substitution, nucleophilic, biomolecular )
SN1 ( substitution, nucleophilic unimolecular)
1. Substitution Nucleophilic Bimolecular SN2
The reaction of methyl chloride and hydroxide ions to produce methanol and chloride ions follows second-order kinetics, which means that the rate of the reaction is determined by the concentrations of both reactants.
To put it another way, rate = k (methyl chloride) (hydroxyl ion).
This rate law implies that both the alkyl halide and the nucleophile (OH) participate in the rate-determining step of the reaction at the same time.
In other words, when the incoming nucleophile (OH ion) approaches the alkyl halide molecule and begins interacting with it, the carbon-halogen bond begins to break, and a new carbon-OH bond begins to form. These two processes occur concurrently in a single step, and no intermediate is formed. Thus, SN2 reactions are concerted reactions that occur in a single step.
As shown below, such reactions occur via a transition state in which both reactants are partially bonded to each other.
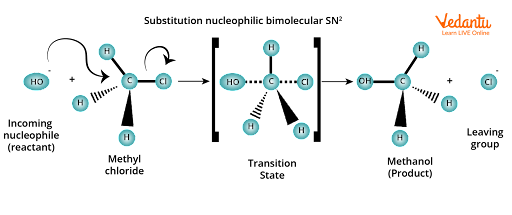
Substitution of nucleophilic bimolecular SN2
In the transition state, the carbon atom is simultaneously bonded to the incoming nucleophile (OH- ion) and the outgoing leaving group (Cl- ion). In other words, because carbon atoms are bonded to five atoms in the transition state, the transition state is unstable and thus cannot be isolated. Due to its instability, it eventually decomposes to form the product methanol and the leaving group (Cl- ion).
It is worth noting that in SN2 reactions, the nucleophile ( OH- ion) attacks from the back side, and the leaving group (i.e., Cl- ion) leaves from the side.
As a result, SN2 reactions are always accompanied by configuration inversion, just as an umbrella turns inside out in a strong wind. Walden Inversion is another name for this inversion of configuration.
2. Substitution Nucleophilic Unimolecular SN1.
SN1 reactions are typically carried out in polar protic (hydroxylic) solvents such as water, alcohol, acetic acid, and so on. The reaction between tert-butyl bromide and the OH ion to produce tert-butyl alcohol and Br ion follows first-order kinetics, which means that the reaction rate is determined solely by the concentration of tert-butyl bromide and is independent of the concentration of the OH- ion. In other words, Rate = ((CH), CBr).
This rate law implies that the reaction takes two steps. Tert-butyl bromide is ionized in the first step, yielding tert-butyl carbocation and a bromide ion.
The energy required to cleave the C-Br bond is obtained by solvating the bromide ion with the proton of protic solvents. This is the reaction's rate-determining step because it is slow and reversible.
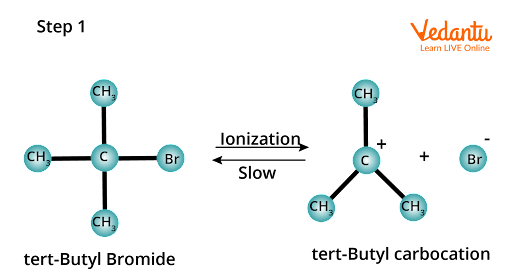
Substitution of nucleophilic unimolecular SN1
In the second step, the carbocation, being a reactive chemical species, is immediately attacked by the nucleophile, OH- ion, yielding the substitution product, tert-butyl alcohol. This step is quick and thus does not affect the rate of the reaction.
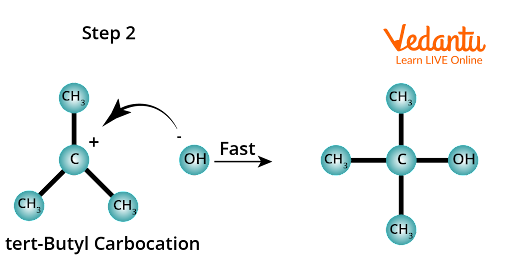
Substitution nucleophilic unimolecular (SN1).
Haloalkanes' Relative Reactivity to SN2 and SN1 Reactions
As previously stated, the nucleophile attacks the a-carbon from the back side in SN2 reactions (i.e., carbon carrying the halogen). As a result, the presence of bulky substituents on or near the carbon atom tends to hinder (or block) the nucleophile's approach to the a-carbon due to steric hindrance, making the reaction difficult to occur. Evidently, the slower the reaction, the greater the steric hindrance.
Since they have three small hydrogen atoms on the a-carbon, methyl halides are the most reactive in reactions of all the alkyl halides. Tertiary alkyl halides, on the other hand, have three halides and one bulky alkyl group. Thus, in SN2 reactions, halo alkyl groups are the least reactive, followed by secondary alkyl halides with two and primary alkyl groups.
Carbocations, on the other hand, are the intermediates in SN1 reactions. The higher the stability of the carbocation, the easier it is to form and, thus, the faster the reaction because the stability of carbocations decreases with increasing order:
As a result, the reactivity of alkyl halides towards SN1 reactions decreases in the same order, i.e., 3° alkyl halides > 2° alkyl halides > 1° alkyl halides > methyl halides.
Conclusion
From the above discussion, we understood what nucleophilic reactions and their types are. Nucleophilic substitution are those reactions in which one nucleophile replaces a weaker nucleophile. There are two types based on the order of the reaction. Both reactions proceed through different mechanisms. Both the reactions have significant uses in the field of chemistry and research.
FAQs on Substitution Reaction and Its Types for JEE
1. What is the nucleophilicity order and what are good leaving groups?
A nucleophile is a substance that donates electron pairs to electrophiles to form chemical bonds with them. The order of nucleophilicity within a group is the inverse of the order of basicity. This order of nucleophilicity is related to the nucleophile's polarizability. The order I > Br > Cl is frequently encountered in the study of reaction mechanisms.
Good leaving groups are weak bases. They are content and secure on their own. Weak bases include halide ions (I-, Cl-, Br-), water (OH2), and sulfonates such as p-toluenesulfonate and methanesulfonate. The stronger the leaving group, the weaker the base.
2. In a nucleophilic substitution reaction, what is the best leaving group and what is the distinction between electrophilic and nucleophilic substitution?
Alkyl chlorides, as well as alkyl bromides and alkyl iodides, are common reactants in laboratory nucleophilic substitution reactions. Iodide, the least basic of the four common halides (F, Cl, Br, and I), is the most effective leaving group.
The main contrast between nucleophilic and electrophilic substitution reactions is that in nucleophilic substitution processes, a leaving group is substituted by a nucleophile, whereas in electrophilic substitution reactions, a functional group is substituted by an electrophile. The hydrolysis of an alkyl bromide, R-Br, under basic environments, where the attacking nucleophile is OH and the leaving group is Br, is an example of a nucleophilic substitution reaction.
























