




Introduction to Carbocation
A carbocation is a cation with a positively charged carbon atom. Some examples are methenium CH3+, methanium CH5+, and vinyl C2H3+ cations. Carbocations containing more than one positively charged carbon can be found very rarely. For example, ethylene dication C2H42+. According to the IUPAC, a carbocation is defined as any cation that contains an even number of electrons with a significant portion of the positive charge residing on a carbon atom. The carbon atom of a carbocation is sp2 hybridised. The three hybridised orbitals of the carbon atom are used for single bonding to three substituents, and the remaining p-orbital is empty.
Carbocations are important reactive intermediates in many organic synthesis reactions. Carbocation rearrangements are generally reactions in organic Chemistry where the movement of a carbocation from an unstable state to a more stable state occurs through the molecular rearrangement in the structure. After the carbocation rearrangement, the new molecule is the structural isomer of the initial molecule.
Classification of Carbocations
Primary Carbocation: Here, the carbon having the positive charge is attached to one carbon of an alkyl or aryl group.
Secondary Carbocation: In this carbon, a positive charge is attached to two other carbons.
Tertiary Carbocation: In this case, the carbon having the positive charge is attached to three carbon atoms.
In methyl carbocation, the carbon bearing a positive charge is attached to only three hydrogen atoms.
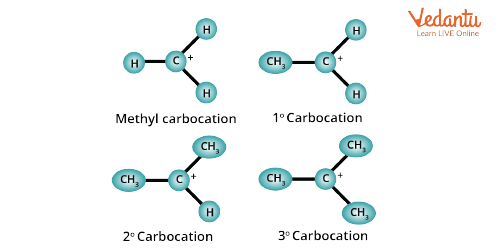
Type of Carbocation
Generation of Carbocation
The different methods available to generate stable or unstable carbocation are:
1. Ionisation: When direct ionisation of an alkyl or aryl compound occurs, a leaving group attached to a carbon atom leaves with its pair of electrons, forming a carbocation. Stable carbocations can be produced in super acids. For example, the addition of alkyl fluorides to SbF5 forms a stable carbonation.
$\mathrm{RF}+\mathrm{SbF}_{5} \rightarrow \mathrm{R}^{+}+\mathrm{SbF}_{6}^{-}$
2. Ionisation After an Initial Reaction: Occasionally, the functional groups in an organic compound may undergo an initial reaction first. During this reaction, the functional group converts to a good leaving group, and the intermediate formed then ionises into a carbocation after the removal of the leaving group. Protonation of organic alcohol generates an oxonium ion which ionises to a carbocation by losing a water molecule.
$\mathrm{R}-\mathrm{OH} \stackrel{\mathrm{H}^{+}}{\longrightarrow} \mathrm{R}-\stackrel{+}{\mathrm{OH}_{2}} \rightarrow \mathrm{R}^{+}+\mathrm{H}_{2} \mathrm{O}$
3. Attack of π-system on Electrophiles: When an electrophile attacks one of the atoms of a π-system in an organic molecule, the adjacent atom acquires a positive charge. In such cases, if the positive atom is carbon, then a carbocation is formed. When the π-system of an alkene or alkyne gets attacked with an electrophile like proton, bromonium ion, etc., a positive charge develops on the adjacent carbon and leads to the formation of a carbocation intermediate. For example,
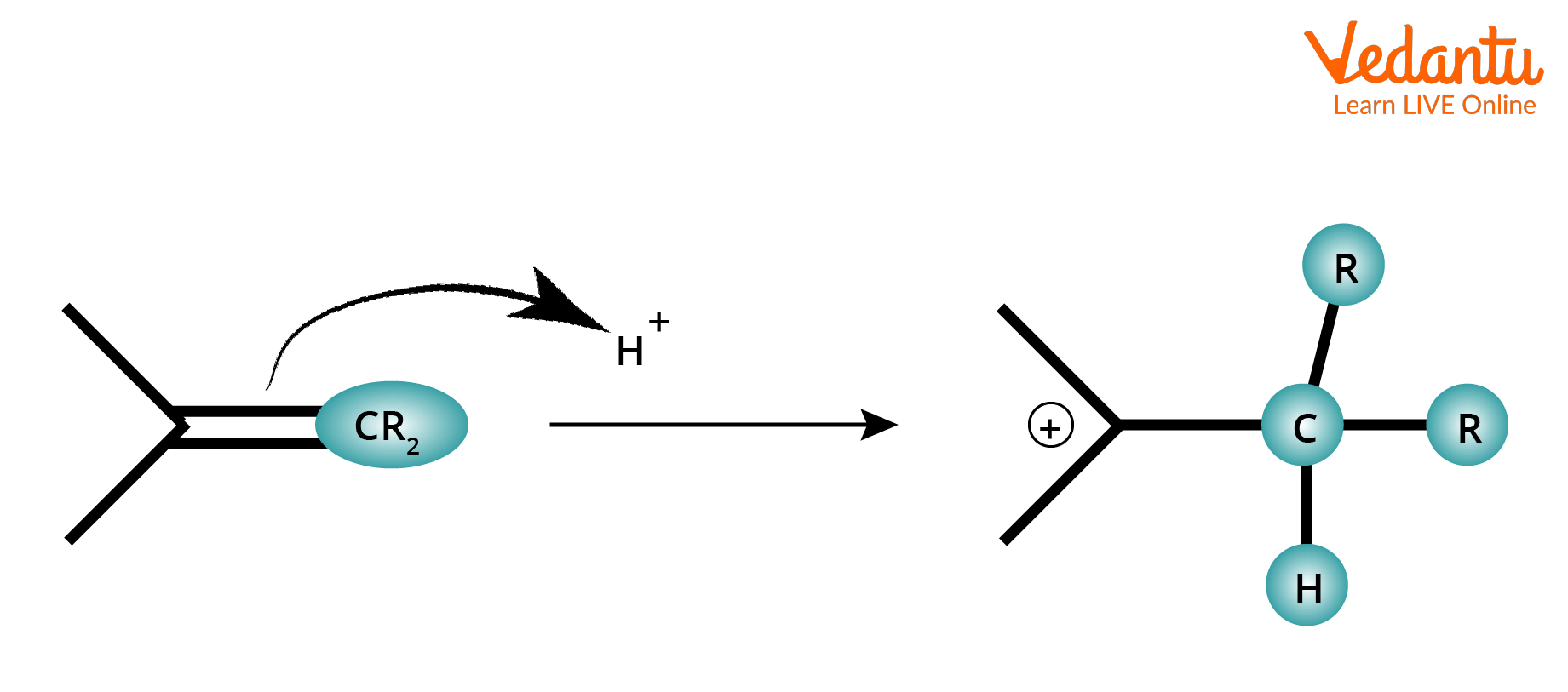
Attack of π-system in Alkene on an Electrophile.
Stability of Carbocation
The stability of a carbocation is decided by three main factors, inductive effect, hyperconjugation, or resonance effect. Carbocations are the most stable when the charge is on a tertiary carbon and the least stable on a primary carbon. The order of stability is 3o carbocation > 2occarbocation > 1o carbocation > Methyl carbocation.
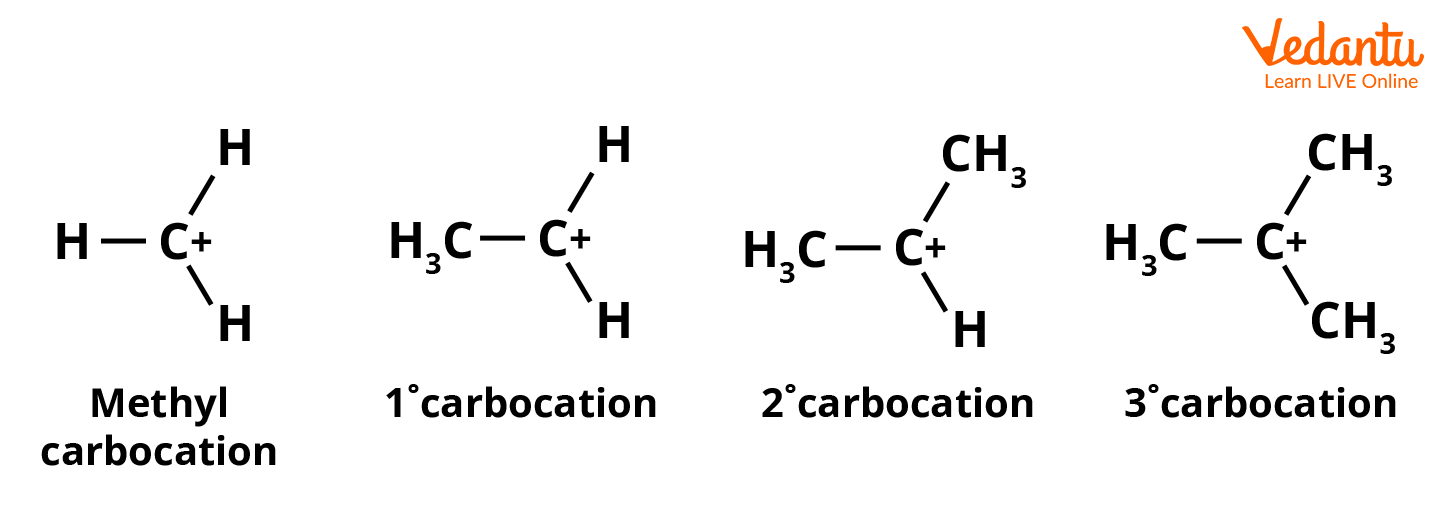
Order of Stability of Carbocation
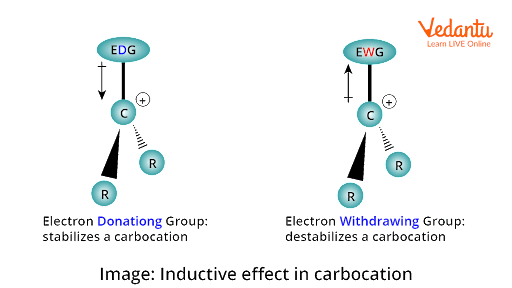
1. Inductive Effect: If the groups attached to positively charged carbon are electron releasing, they will delocalise the positive charge on carbon and will decrease the intensity of the positive charge on carbon. This stabilises the carbocation. The more alkyl groups are attached, the more stable the carbocation will be as alkyl groups release electrons.
2. Hyperconjugation: If in any carbocation, the sigma bond in conjugation with vacant p orbital on carbon has a positive charge, then it participates in delocalisation. Thus, they spread the charge all over the alkyl groups. This effect is known as hyperconjugation or no bond resonance. The structure of the ethyl carbocation has the following series of resonance forms, according to the theory of hyperconjugation.
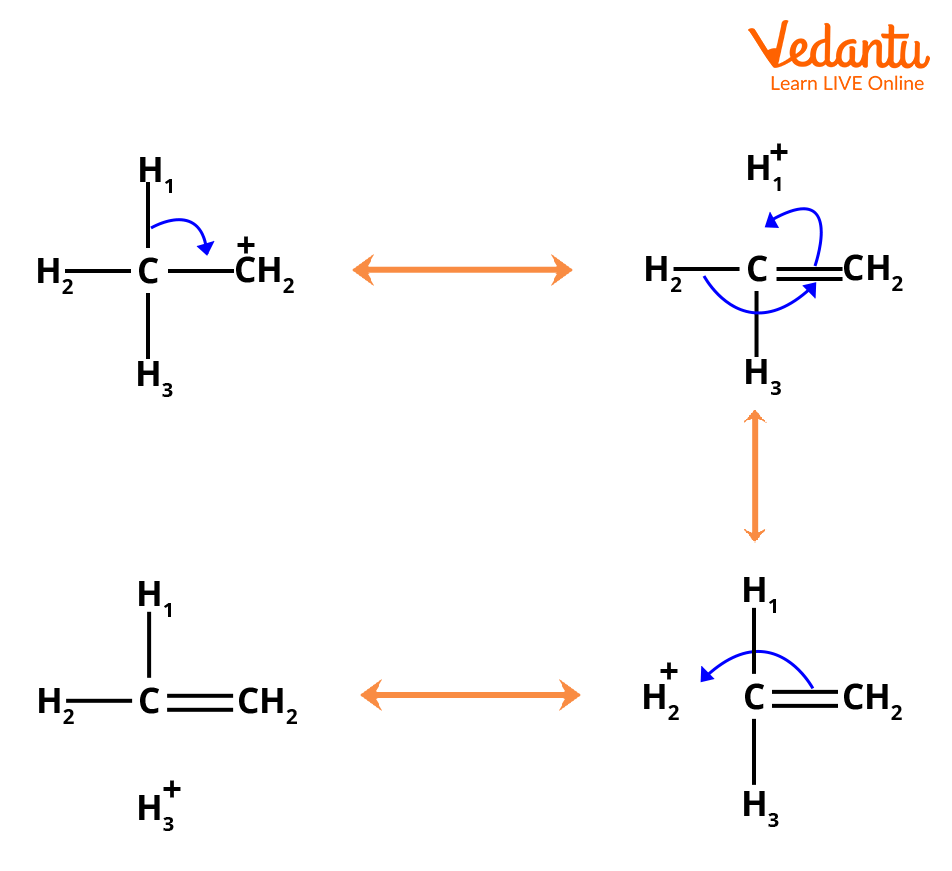
Resonating Structure of Ethyl Carbocation
(Note: in the above image, numbers in subscript don’t indicate the number of atoms but used to distinguish between hydrogen atom.)
3. Resonance: Conjugation with multiple bonds or lone pairs of electrons increases the stability of a carbocation. For example, triphenylmethyl cation has higher stability than diphenylmethyl cation. In the case of triphenylmethyl cation, delocalisation of positive charge is more as it has more resonating structures.
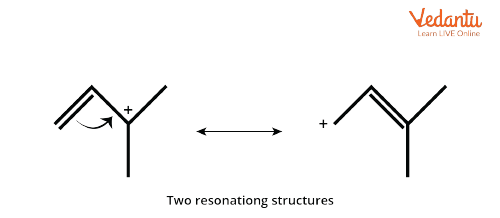
Resonance Effect in Carbocation
Rearrangement of Carbocation
Carbocation rearrangements are the movement of a carbocation from an unstable state to a more stable state through structural rearrangement to form a structural isomer of the initial molecule. Rearrangements of carbocation are of two types: hydride shift and alkyl shift. When rearranged, the molecules can also undergo unimolecular substitution (SN1) or unimolecular elimination (E1) reactions.
Hydride Shift
In hydride shift, hydrogen moves over from one carbon to a neighbouring, which is less substituted carbon. Here, the hydrogen migrates towards the positively charged carbon with its electron pair, leaving a stable carbocation behind. Generally, hydride shift occurs in the reaction of an alcohol with HBr, HCl, and HI. Examples of carbocation rearrangement are given below.
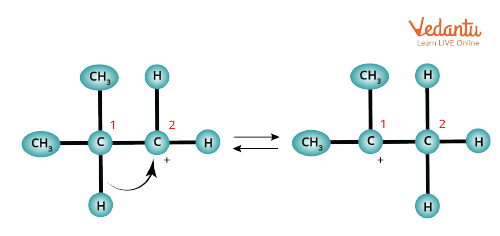
1-2 Hydride Shift
Below is a carbocation rearrangement example, which is a reaction between an alcohol and hydrogen chloride.
Protonation and Loss of Water Molecule: In this SN1 reaction, -OH which leaves the organic alcohol forms a carbocation on carbon (C-3) after receiving a proton from the nucleophile to produce an alkyloxonium ion.
Hydride Shift: The hydrogen atom attached to the carbon atom adjacent to the original carbon (C-2) undergoes a hydride shift in this case.
Attack of Cl Atom: The Cl atom can now attack the carbocation and form a more stable structure because of hyperconjugation. The carbocation here is the rearranged most stable carbocation, which is tertiary carbon.
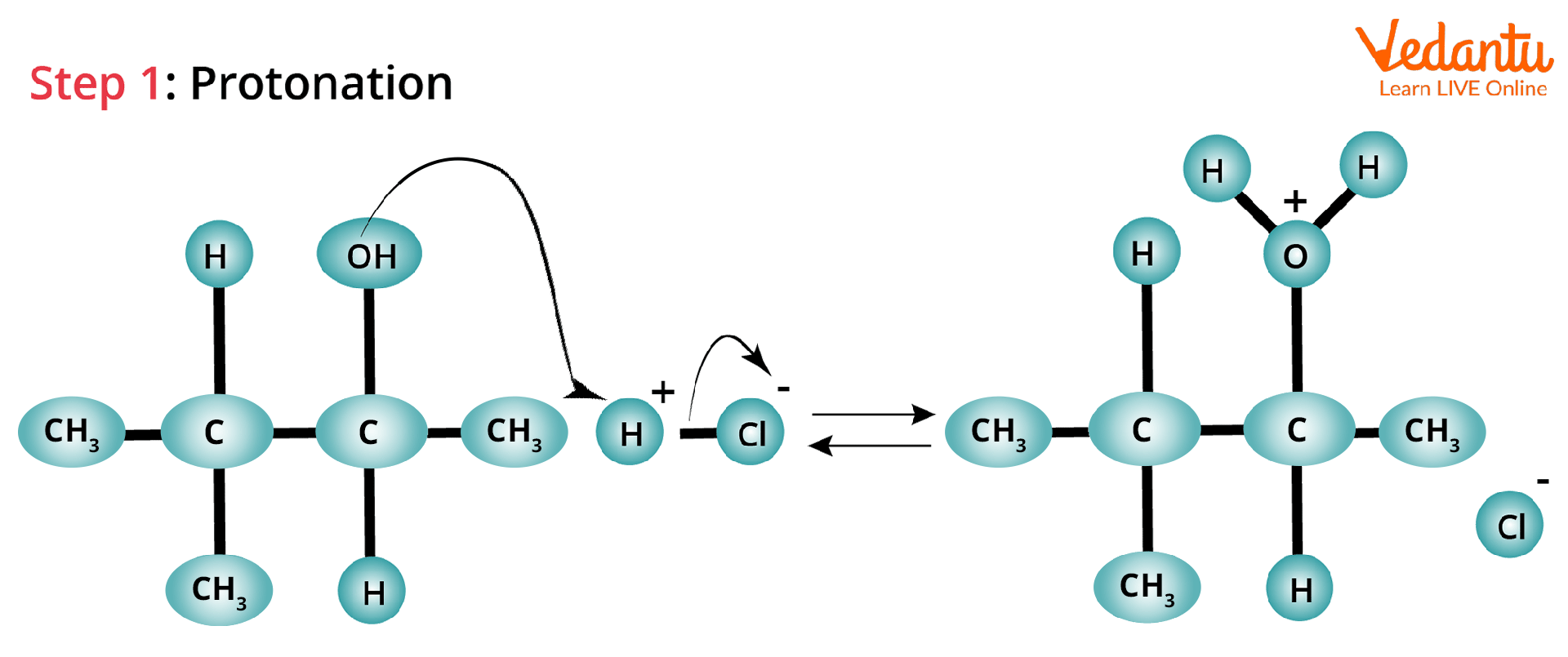
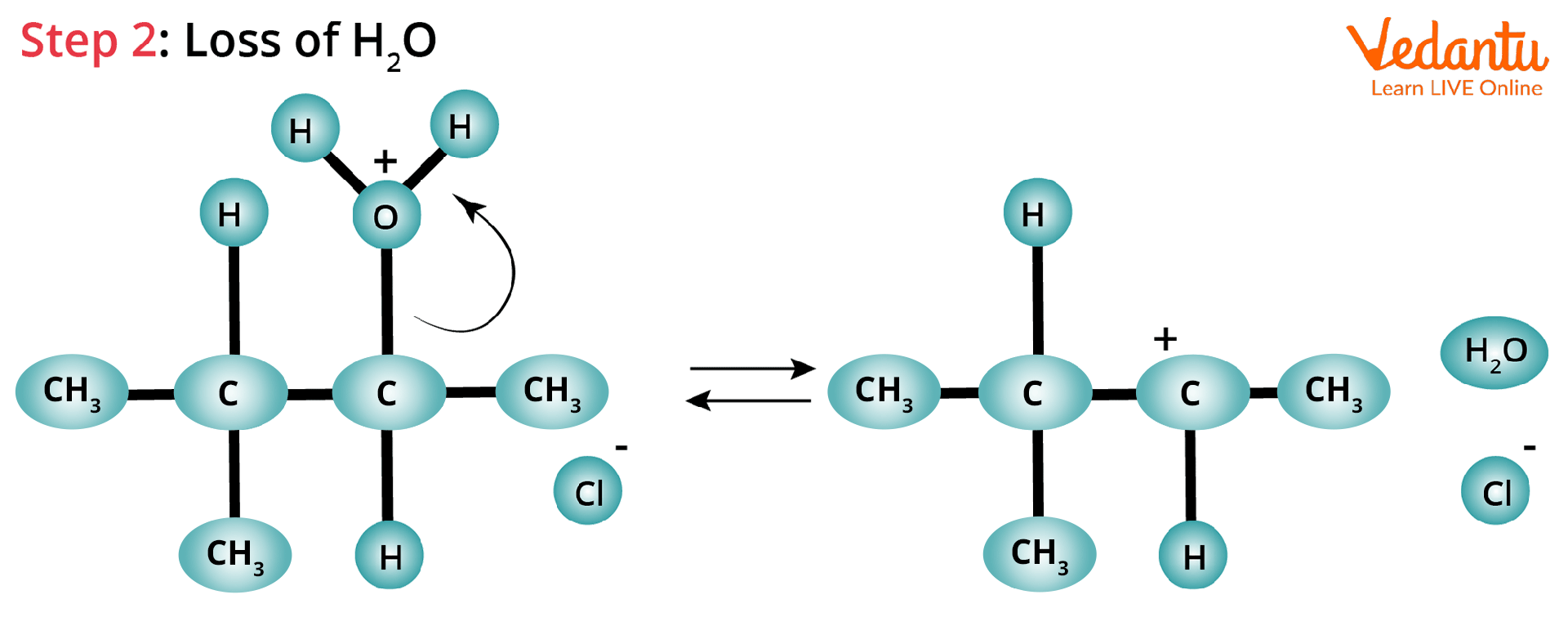
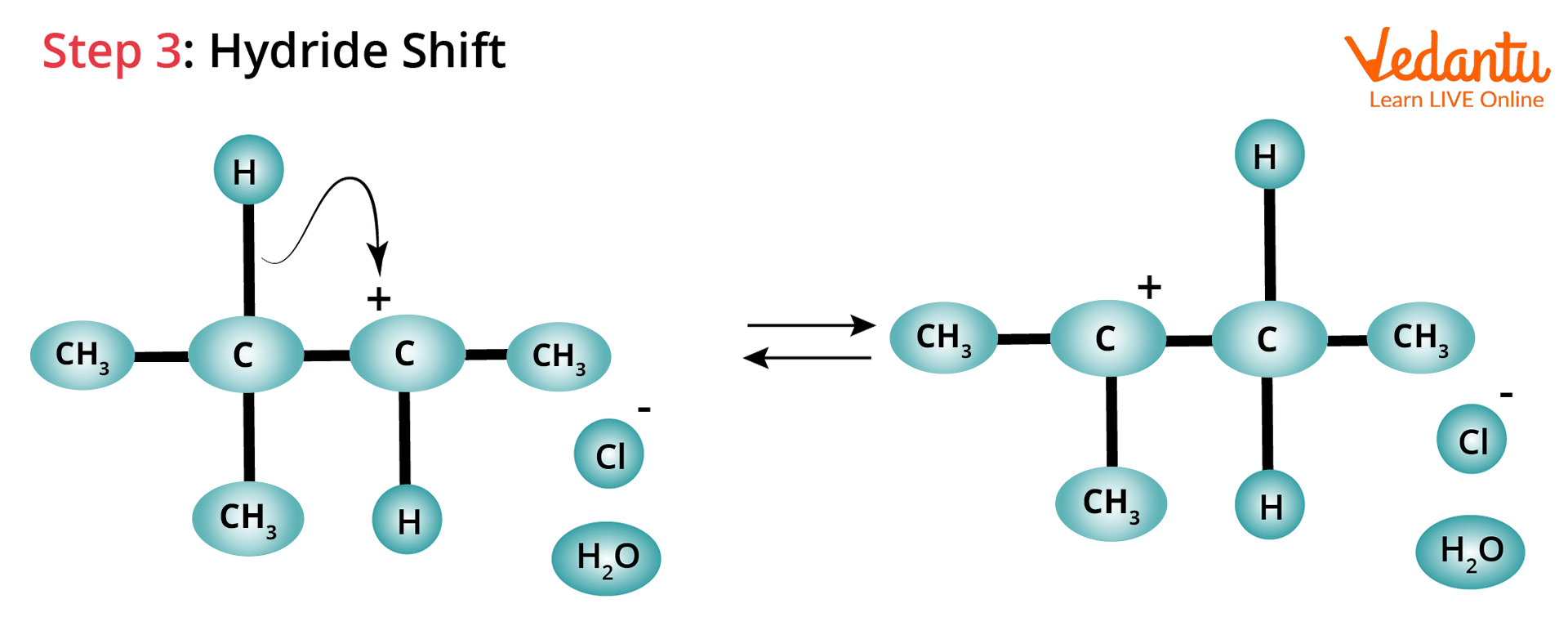
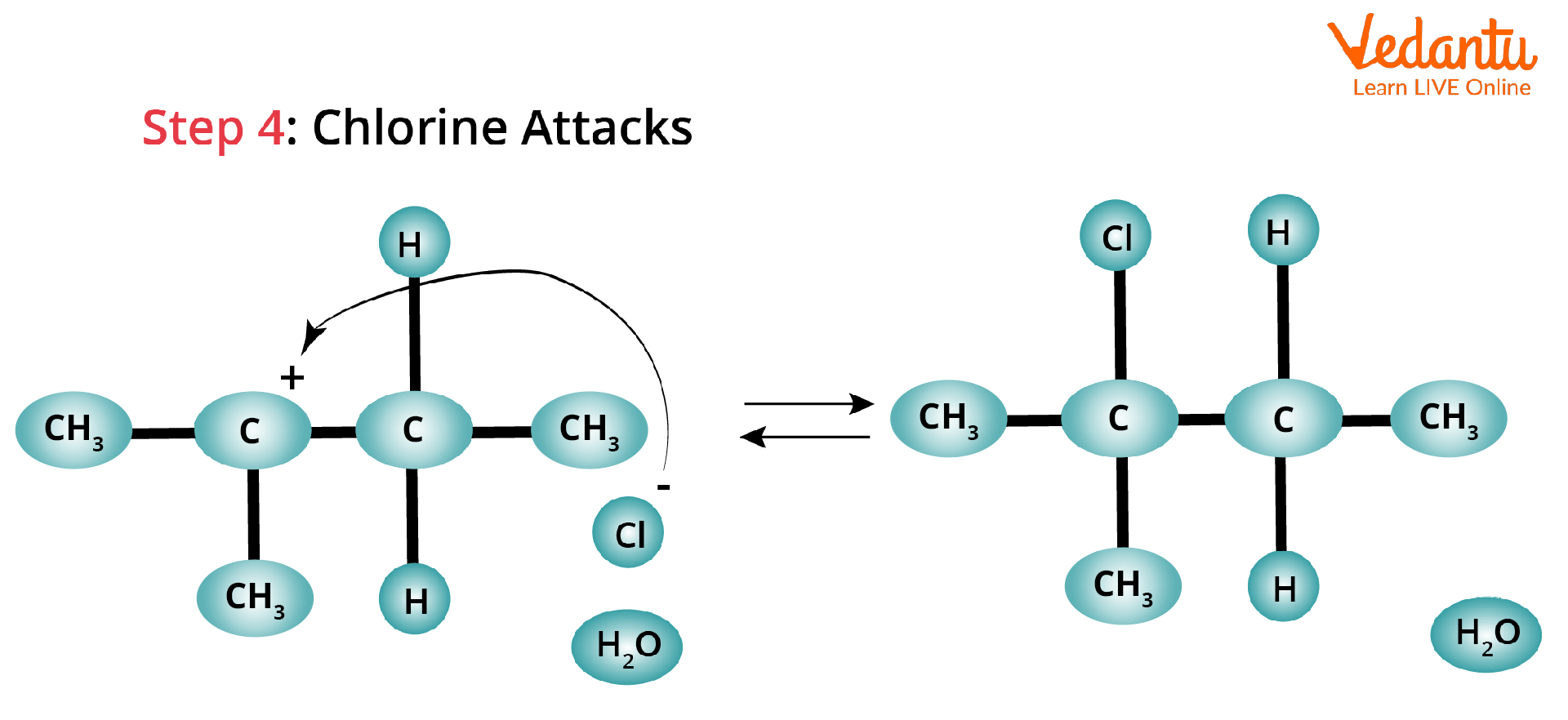
Hydride Shift Rearrangement of Carbocation
Alkyl Shift
Alkyl Shift reactions are similar to hydride shifts. Here, instead of the proton, the alkyl group shifts with the nucleophile. The group that shifts takes its electron pair with it to form a bond with the nearby carbocation. Finally, the shifted alkyl group and the carbocation with positive charge switch positions on the molecule.
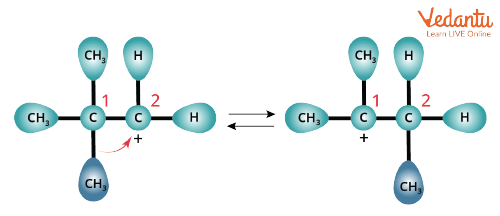
Alkyl Shift Rearrangement of Carbocation
Conclusion
A carbocation, which was earlier known as carbonium, is an ion with a positively charged carbon atom. They are reactive intermediates, an electron deficient species with a sextet of electrons. We can obtain stable carbocations in super acids. The order of stability is tertiary carbocations > secondary carbocations > primary carbocations > methyl carbocation.
The stability of the carbocation can be ensured by factors like inductive effect (+I), hyperconjugation, and resonance, which can decrease the positive charge on carbon. Carbocations generally undergo two types of rearrangements: hydride shift and alkyl shift. The rearranged carbocations can also undergo reactions like unimolecular substitution (SN1) or unimolecular elimination (E1) and form simple or complex mixtures of products.
FAQs on Rearrangement of Carbocation for JEE
1. Discuss the reactivity of carbocations.
In general, carbocations undergo three types of reactions, which are listed below:
Nucleophile Capture: Carbocations will react with even mild nucleophiles like water to form a new bond. The carbocation may combine with a negative ion or species possessing an electron pair (nucleophiles such as OH-, Cl-, Br-, water ) and they are very fast reactions.Elimination to form a pi Bond: Carbons near the carbocation can lose a proton to form a double or triple bond with the carbocation.
2. What is rearrangement in carbocation?
The migration of a carbocation from an unstable state to a more stable state is known as a carbocation rearrangement and is a common phenomenon in organic Chemistry. Carbocation rearrangements can be of two different types: hydride shifts and alkyl shifts. Hydride Shift indicates that a hydrogen moves from one carbon to a nearby carbocation that has less substitution. Alkyl shift refers to when an alkyl group moves and takes its electron pair along with it to create a bond with a nearby carbocation.
























