




Reactions Involving Addition of Water
Reactions involving the addition of water, also known as hydration reactions, are chemical reactions that involve the combination of a substrate or substance with water. It is basically the addition of water to different compounds that produce economically important products. In these types of reactions, water is one of the reactants and is converted into a product. The most common hydration reactions are the hydration of alkenes and alkenes that yield alcohol. This method is extensively employed in the industrial production of butanol, isopropanol, etc.
Addition of Water to Alkene with Example
Alkenes are organic compounds having a double bond between the carbon atoms (C=C). They are unsaturated hydrocarbons with the general formula CnH2n. Alkenes are also called olefins because upon reaction with bromine or chlorine, they are capable of forming oil-like products.
Upon the addition of water to alkenes in the presence of an acidic medium, such as concentrated sulphuric acid, the formation of alcohol takes place.
$\mathrm{R}-\mathrm{CH}=\mathrm{CH}_{2}+\mathrm{H}_{2} \mathrm{O} \xrightarrow{\text { conc } \mathrm{H}_{2} \mathrm{SO}_{4}}\mathrm{R}-\mathrm{CH}(\mathrm{OH})-\mathrm{CH}_{3}$
This addition reaction is in accordance with Markovnikov's rule.
Markovnikov's rule states that whenever an unsymmetrical molecule is added to an unsymmetrical alkene, the positive part of the molecule being added will get added to the carbon atom having the higher number of hydrogen atoms.
Example: Propene reacts with water to form isopropyl alcohol according to Markovnikov's rule.
$\mathrm{CH}_{3}-\mathrm{CH}=\mathrm{CH}_{2}+\mathrm{H}_{2} \mathrm{O} \xrightarrow{\mathrm{conc}~\mathrm{H}_{2} \mathrm{SO}_{4}}\mathrm{CH}_{3}-\mathrm{CH}(\mathrm{OH})-\mathrm{CH}_{3}$
Addition of Water to Alkyne with Example
Alkynes are unsaturated hydrocarbons. They are aliphatic in nature and are open chain organic compounds. They have a triple bond present in carbon atoms ($\mathrm{C} \equiv \mathrm{C}$) with the general formula CnH2n-2.
The addition of water to alkynes occurs in acidic medium. Only a single mole of water is added to an alkyne upon heating the mixture up to 333K with dilute sulphuric acid in the presence of mercuric sulphate. This results in the formation of an enol (vinyl alcohol) intermediate, which is very unstable. This enol then undergoes tautomerization, forming carbonyl compounds such as ketones or aldehydes. Ketones are formed when water is added to terminal alkynes, whereas aldehydes are formed when water is added to nonterminal alkynes.
Example: Addition of water to ethyne. This follows Markovnikov's rule.
$\underset{\text{Ethyne}}{\mathrm{HC} \equiv \mathrm{CH}}+\mathrm{H}_{2} \mathrm{O} \underset{\mathrm{HgSO}_{4}}{\xrightarrow{\mathrm{H}_{2} \mathrm{O}}} \mathrm{H}_{2} \mathrm{C}=\mathrm{CH}(\mathrm{OH}) \xrightarrow{\text { Tautomerization }} \underset{\text{Acetaldehyde}}{\mathrm{H}_{3} \mathrm{C}-\mathrm{CHO}}$
Mechanism:
Step 1: Electrophilic addition of mercuric ion (Hg2+) or mercury (II) ion to alkyne, which results in the generation of mercury containing vinylic carbocation intermediate.
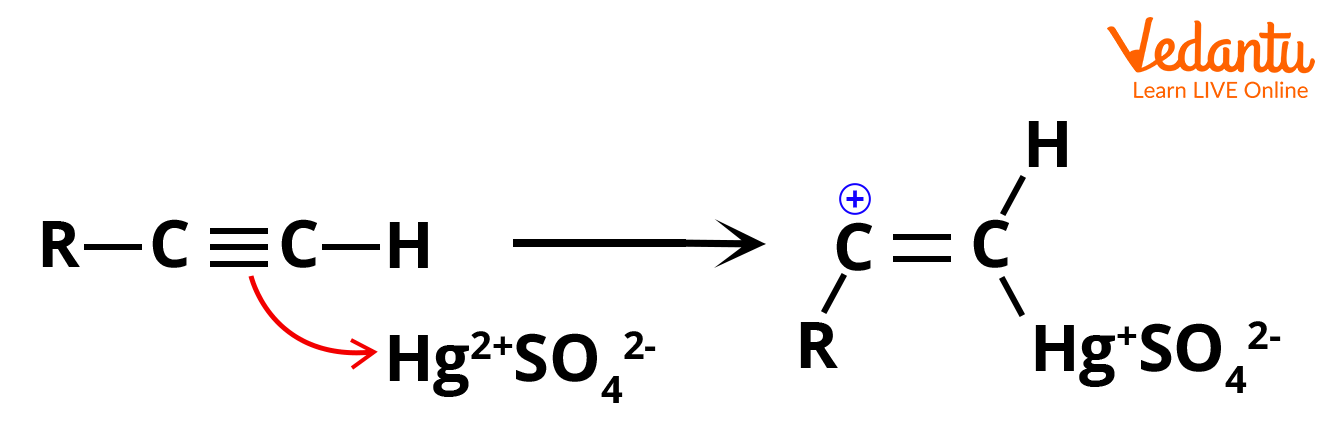
Electrophilic Addition of Hg2+
Step 2: Nucleophilic attack by water on the carbocation which converts it to a single bond (C-O bond) to generate protonated enol, also known as oxonium ion.
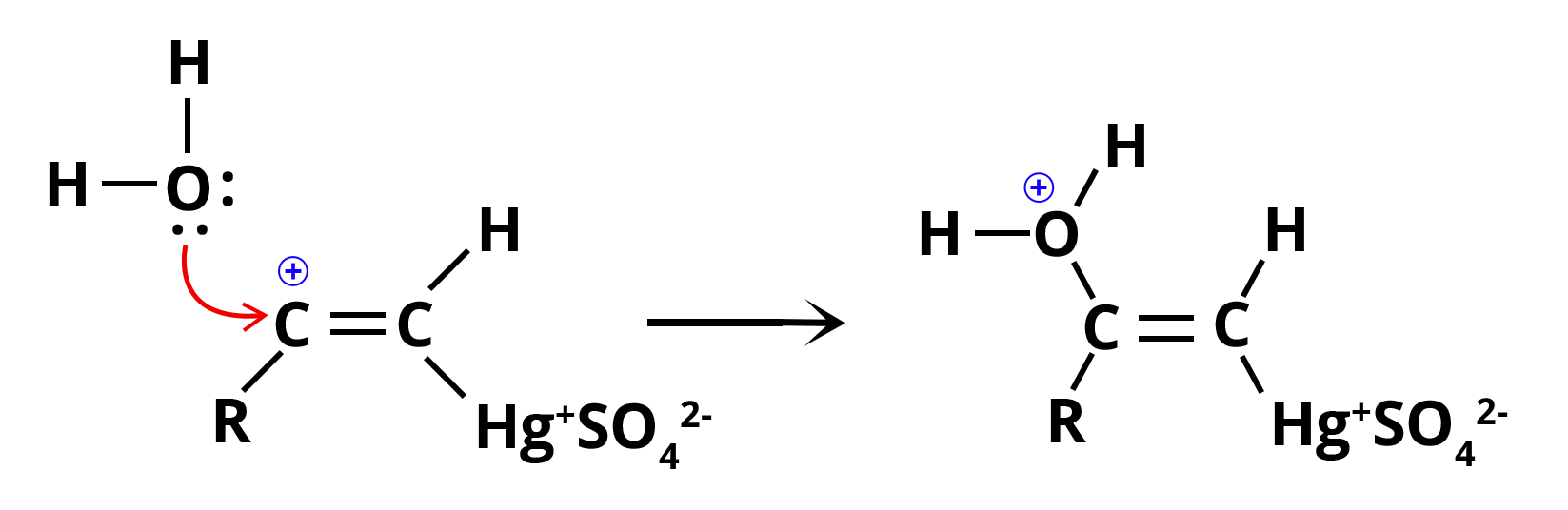
Nucleophilic Attack by Water
Step 3: Water causes proton loss (deprotonation) in enol, resulting in vinyl alcohol precursor, also known as organomercury enol. The primary purpose of this deprotonation reaction is to stabilise the enol.
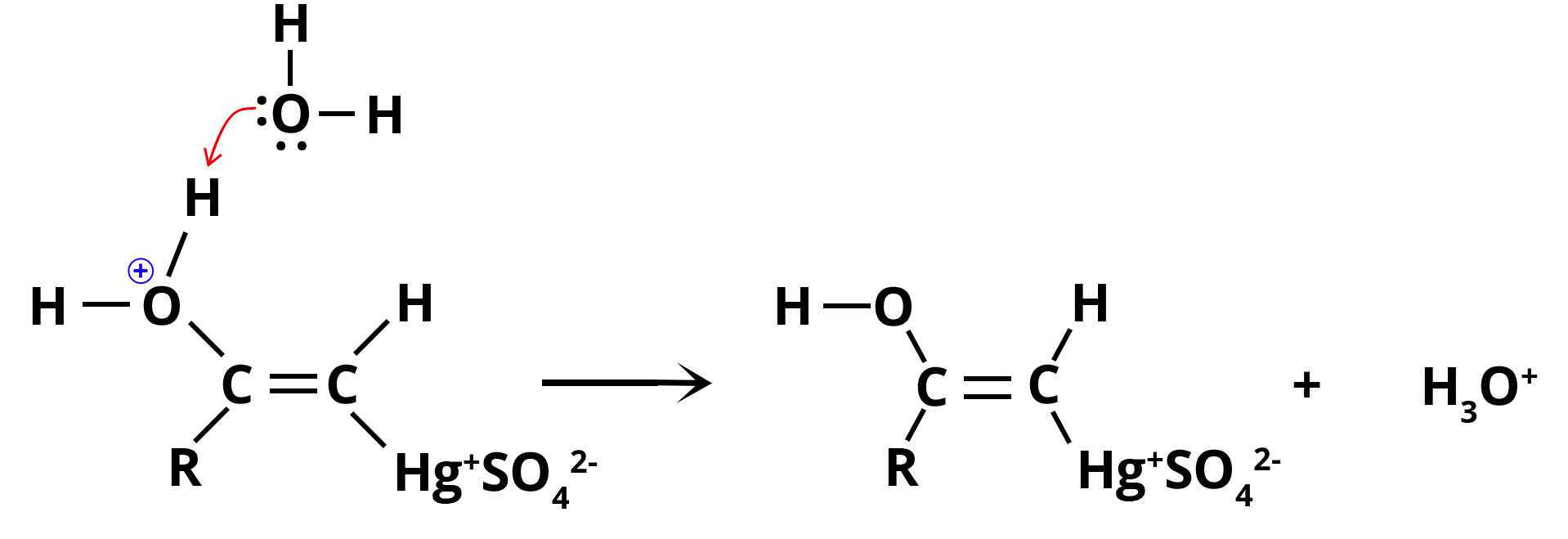
Deprotonation of Enol by Water
Step 4: Substitution of mercury with H+ ion from the hydronium ion, which generates neutral enol and also regenerates Hg2+ catalyst.
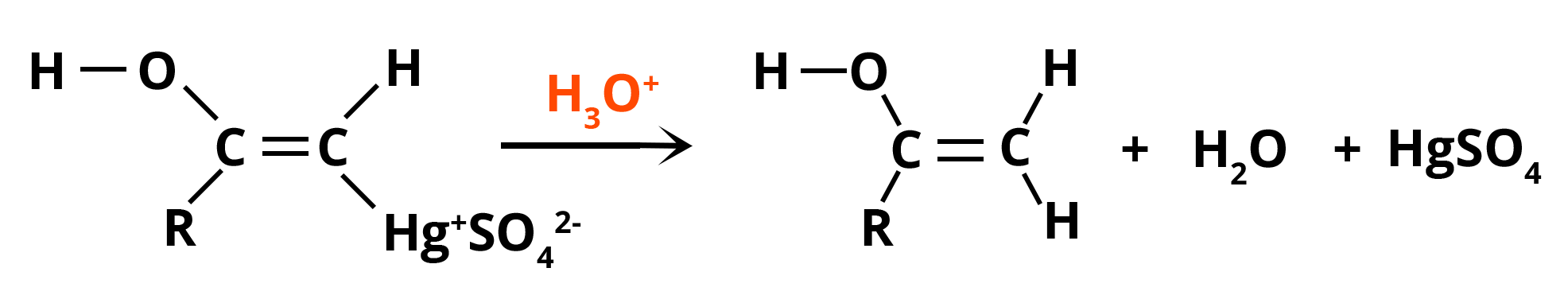
Substitution by Hydronium Ion
Step 5: Enol is converted to ketone via keto-enol tautomerization (similarly, less stable intermediate produces aldehyde).
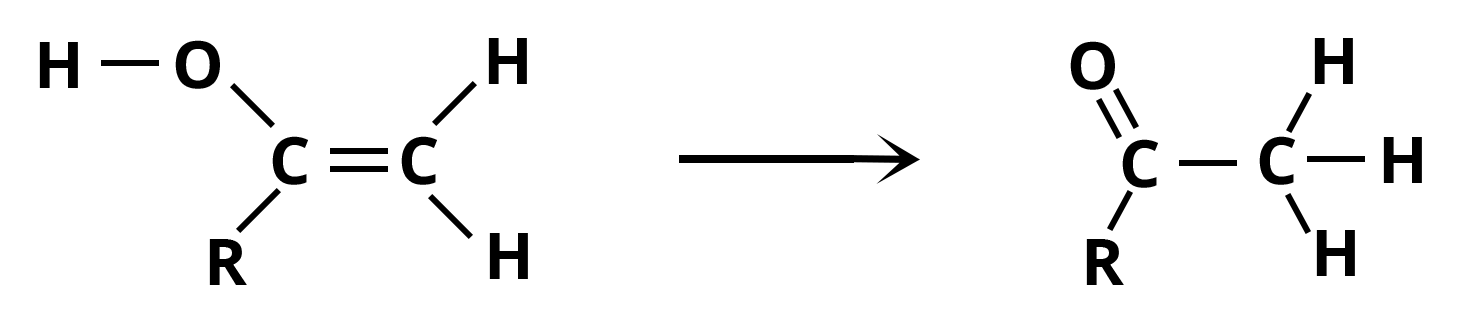
Tautomerization
Representation of the overall addition reaction mechanism:
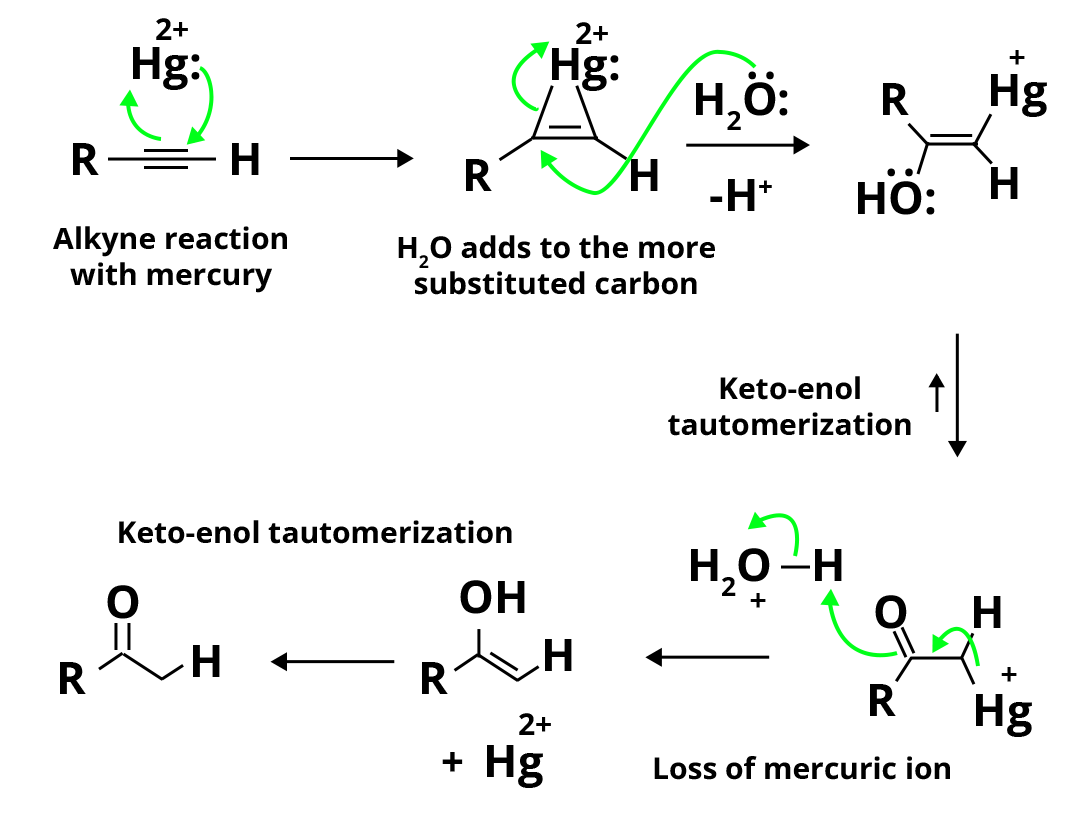
Overall Mechanism Process
Addition of Water to BiCl3 (Bismuth Chloride)
Upon addition of water to BiCl3, it is readily hydrolyzed to form a white precipitate of bismuth oxychloride (BiOCl). This is a reversible reaction. It can be reversed by the addition of an acid such as hydrochloric acid (HCl), which again leads to the formation of a clear solution.
$\mathrm{BiCl}_{3}+\mathrm{H}_{2} \mathrm{O} \rightleftharpoons \mathrm{BiOCl}+\mathrm{H}_{2} \mathrm{O}$
When water vapour reacts with BiCl3 at a temperature less than 50℃, an intermediate is formed instead of bismuth oxychloride (BiOCl). This intermediate is bismuth monohydrate (BiCl3.H2O).
Addition of Water to Quick Lime
Calcium oxide (CaO) is most commonly referred to as quicklime. It is primarily derived from limestone (also known as calcium carbonate (CaCO3). It is white in colour and is alkaline in nature.
When the addition of water to quicklime takes place, there is the formation of calcium hydroxide, also called slaked lime, accompanied by the generation of heat. Therefore, it is an exothermic reaction. Thus formed product Ca(OH)2 is sparingly soluble.
$\mathrm{CaO}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{Ca}(\mathrm{OH})_{2}$
Conclusion
The addition of water to various compounds has different types of reactions on them and produces a wide variety of products. Hydration reactions are those reactions where different substrates are combined with water.
The addition of water to alkenes yields alcohol. This reaction takes place in the presence of sulphuric acid and in accordance with Markovnikov's rule. The addition of water to an alkyne results in the formation of carbonyl compounds such as aldehydes and ketones. This reaction involves the formation of an enol (vinyl alcohol) intermediate. The addition of water to BiCl3 forms a white precipitate of bismuth oxychloride (BiOCl). The addition of water to quicklime forms sparingly soluble slaked lime, i.e., calcium hydroxide.
FAQs on Hydration Reactions Along with Examples for JEE
1. Why do alkynes exhibit an acidic nature? Are they strong acids or weak acids?
Alkynes have at least one bond. Here, the carbon atom is sp hybridised. Due to the large percentage (50%) of s character in alkynes, which makes the sp hybrid orbitals of the carbon atom highly electronegative, they can significantly attract the C-H bond of alkynes towards themselves. Thus, they are capable of losing hydrogen atoms to form alkynide ions. Therefore, they behave as Bronsted Lowry acids.
All alkynes, however, are weak acids, or in other words, slightly acidic. However, their conjugate bases are strong, making them really great nucleophiles.
2. Why are alkanes insoluble in water but are readily soluble in organic solvents?
Alkanes are not soluble in water because water is highly polar, whereas alkanes are nonpolar in nature. We know that ‘‘like dissolves like’’, therefore making alkanes insoluble in water. The intermolecular hydrogen bonds tightly bind one water molecule to another water molecule, making it difficult for the alkanes to get between these strong bonds. Hence, water does not dissolve alkanes.
However, alkanes are soluble in organic solvents such as ether and chloroform. This is because organic solvents lack intermolecular hydrogen bonding and the polarity of organic solvents is extremely low, making it easy for the alkanes to dissolve in them.


































