




What is an Electrophilic Addition Reaction?
Alkenes are unsaturated hydrocarbons, which means that the compound has at least one double bond. They show electrophilic addition reactions in which an electrophile attacks the carbon-carbon double bond to create additional products due to the presence of pi electrons. Electrophilic addition reaction examples include the addition of alkyl halide to the alkene. Electrophilic addition reactions occur in a wide range of alkenes. Electrophilic addition reactions of alkenes include the addition of hydrogen halides such as hydrogen bromide and hydrogen chloride to alkenes, resulting in the formation of alkyl halide. HI > HBr > HCl is the usual trend for the addition of hydrogen halide. A double bond donates electron pairs to electrophiles approaching it.
Electrophilic Addition of HX to Alkene
The addition reaction to the C=C double bond is the most common type of alkene reaction. As a result of the addition process, a tiny molecule is added to a multiple bond, and one bond is transformed into two bonds (unsaturation degree reduces). The addition reaction is the opposite of the elimination reaction.
A hydrogen halide reacts with an alkene to form an alkyl halide as a result.
Breaking a carbon to carbon double bond is followed by the electrophilic addition of a hydrogen atom and halogen in the hydrohalogenation of alkenes. According to Markovnikov's rule, the halide will add to the more substituted carbon. A haloalkane, commonly known as an alkyl halide, is the end product of the reaction.
(Image will be uploaded soon)
Addition of a Hydrogen Halide to Alkene
Mechanism of Hydrohalogenation of Alkenes
Attack of Electrophile on Alkene
The H in the HBr electrophile is attacked by the two pi electrons from the double bond. Between the hydrogen from HBr and carbon from the double bond, the two pi electrons form a C-H sigma bond. The electrons from the H-X bond transfer onto the halogen at the same time, forming a halide anion. One of the carbons in alkene becomes an electron-deficient carbocation intermediate when pi electrons are removed from the double bond. The positive charge is held in an unhybridised p orbital on this carbon, which is sp2 hybridised.
(Image will be uploaded soon)
Attack of H on Alkene
Halide Ion Attack as a Nucleophile
The nucleophilic halide anion can now act as an electron donor and attack the generated carbocation, which can now behave as an electrophile. To create the neutral alkyl halide product of electrophilic addition mechanism, the electron pair forms an X-C sigma bond(C-Br) in this case.
(Image will be uploaded soon)
Attack of Halide Ion on Carbocation
Energy Diagram of Reaction
The diagram shows the energy profile or path of the reaction. The two peaks represent two transition states. Hence, the reaction is a two-step mechanism. A valley between two peaks is due to the formation of a stable carbocation. As the activation energy of the first step is larger, it is the slow rate-determining step. In the first step of the mechanism, both the alkene and the hydrogen halide are reactants; this electrophilic addition is a second order reaction, and the rate law expression can be written as rate = k(Alkene)(HX). Furthermore, any structural characteristic that can stabilise the transition state between the reactants and the carbocation intermediate reduces E1 and hence increases the reaction rate. The alkyl halide product of this reaction is more stable than the reactants, resulting in an exothermic reaction.
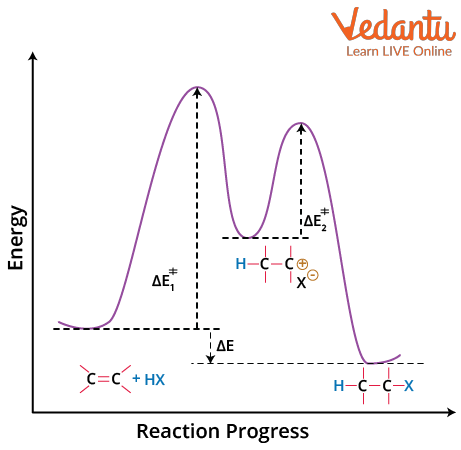
Energy Profile Diagram
Rate of Reaction
When Halogen is Changed
The reaction rates increase in the following order: HF ﹤ HCl ﹤ HBr﹤ HI. Because hydrogen fluoride interacts much more slowly than the other three, it is sometimes ignored in the discussion of these interactions.
The hydrogen-halogen bond must be broken when hydrogen halides react with alkenes. As you move from HF to HI, the bond strength drops, and the hydrogen-fluorine bond becomes particularly strong. Since it's difficult to break the bond between hydrogen and fluorine, adding HF takes a long time.
When Alkene is Changed
The activation energy of the reaction is determined by the stability of the intermediate ions. The activation energy for the reaction decreases as you progress to more complex alkenes. As a result, the reactions become quicker. More stable the carbocation formed faster will be the reaction.
Conclusion
Electrophilic addition reactions occur in a wide range of alkenes. Electrophilic addition to alkenes includes the addition of hydrogen halides such as hydrogen bromide and hydrogen chloride to alkenes, resulting in the formation of alkyl halide. HI > HBr > HCl is the usual trend for the addition of hydrogen halide. The addition reaction to the C=C double bond is the most common type of alkene reaction. As a result of the addition process, a tiny molecule is added to a multiple bond, and one bond is transformed into two bonds (unsaturation degree reduces). The addition reaction is the opposite of the elimination reaction.
FAQs on Addition of HX to Alkene for JEE
1. What is the Markovnikov rule?
When a protic acid (HX) is introduced to an asymmetric alkene, the acidic hydrogen connects to the carbon atom with the most hydrogen substituents, while the halide group binds to the carbon atom with the most alkyl substituents. The simplified version of the rule says, "Hydrogen is added to carbon with the most hydrogens, and halide is added to carbon with the least hydrogens." Hydration of alkene is another example of the Markovnikov rule. This rule is useful in predicting the outcome of addition reactions.
2. What is the rearrangement of carbocation?
According to the definition of carbocation rearrangements, it is "the migration of the carbocation from an unstable state to a more stable one by utilising various structural reorganisational shifts within the molecule." When a carbocation lacks a hydrogen atom that is present on the next carbon atom and is readily available for rearrangement, an alkyl shift occurs. When a carbocation is produced from an alkyl halide, alcohol, or alkene, the carbocation may undergo rearrangement. A hydride shift and an alkyl shift are the two types of carbocation rearrangements.
3. What is the difference between Markonikov and anti-Markovnikov rule?
Following are the distinctions between Markonilov and anti-Markovnikov rules:
The proton is added to the carbon atom with the greatest number of hydrogen atoms attached in an alkene or alkyne, according to the Markonikov rule. In contrast, the anti-Markovnikov rule states that the proton is added to the carbon atom with the fewest hydrogen atoms attached in an alkene or alkyne.
The hydrogen atom is joined to the carbon atom with the greatest number of hydrogen substituents under the Markovnikov rule, however, under the anti-Markovnikov rule, it is bonded to the carbon atom with the greatest number of hydrogen substituents.
























