




Mechanism of Addition of Ammonia and Its Derivatives to Aldehydes and Ketones
The Addition of Ammonia and Ammonia Derivatives is a fundamental organic reaction that every JEE aspirant should master for scoring in carbonyl chemistry. This process involves the nucleophilic addition of ammonia (NH3) or its organic derivatives to aldehydes or ketones, yielding imines or related compounds important in both analysis and synthesis. Understanding the stepwise mechanism, the role of optimal pH, and how different derivatives react is central for solving mechanism, MCQ, and numericals in JEE Main. Mastery of this concept not only clarifies reaction behaviour but aids in the rapid identification of aldehydes and ketones in complex organic mixtures.
Addition of Ammonia and Ammonia Derivatives: Core Concept
The addition of ammonia and ammonia derivatives to carbonyl compounds is classified as a nucleophilic addition reaction. The strongly polar C=O bond of aldehydes and ketones attracts nucleophiles such as ammonia, which adds to the electrophilic carbon, ultimately converting the sp2 carbon to sp3 and forming a tetrahedral intermediate. On dehydration, this yields imines (Schiff bases) or special derivatives used for purification and identification. These reactions are heavily tested in JEE Main for their selectivity and mechanism.
Ammonia and Key Ammonia Derivatives
Ammonia (NH3) is a trigonal pyramidal molecule with a lone pair of electrons on nitrogen. Its derivatives are produced when one or more hydrogens are substituted by groups like alkyl, aryl, hydroxyl, or acyl. Recognising these derivatives is essential for JEE mechanism tracing and assertion-reasoning questions.
- Hydroxylamine (NH2OH): Forms oximes with carbonyls, popular for identification.
- Hydrazine (NH2NH2): Yields hydrazones, key in qualitative organic analysis.
- Phenylhydrazine (C6H5NHNH2): Used for the formation of phenylhydrazones and osazones (especially with sugars).
- 2,4-Dinitrophenylhydrazine (DNPH): Standard derivative—gives distinctive orange/yellow precipitate for carbonyl detection.
- Semicarbazide (NH2CONHNH2): Yields semicarbazones, aiding purification of aldehydes/ketones.
These derivatives are typically colourless solids or form coloured derivatives with sharp melting points, often used for qualitative detection and purification in labs and JEE problems.
Mechanism of Addition of Ammonia and Its Derivatives
The mechanism of addition of ammonia and its derivatives to carbonyls involves nucleophilic attack followed by dehydration. The carbonyl oxygen is first protonated (by weak acid), increasing carbon electrophilicity. Then, the lone pair on ammonia (or its derivative) attacks the carbonyl carbon, yielding a tetrahedral intermediate. Proton transfer and subsequent dehydration produce the imine (or analogous derivative) and water. The reaction is reversible and the removal of water helps drive it forward.
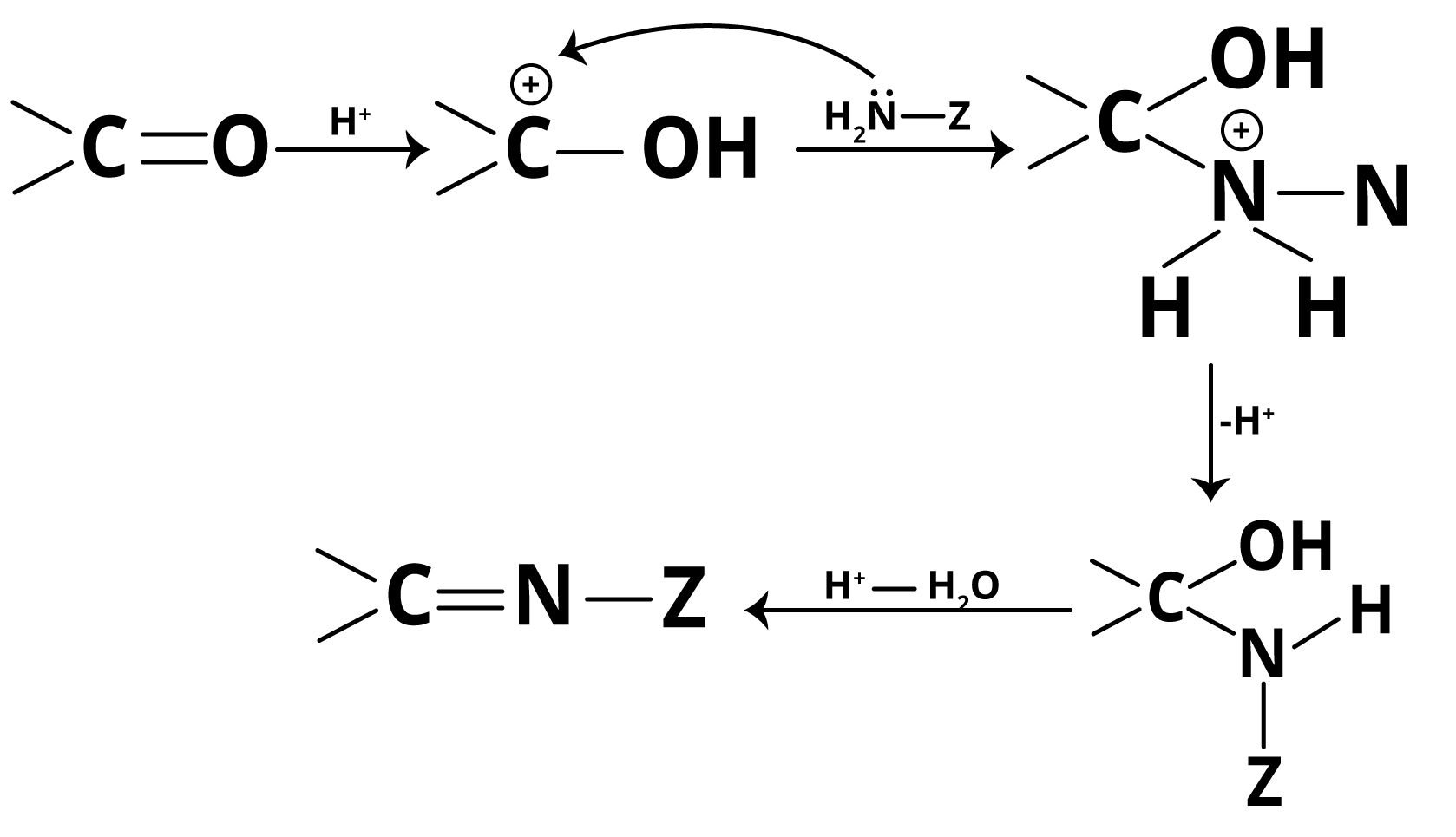
Notably, excessive acid converts ammonia derivatives into non-nucleophilic ammonium salts, halting the reaction. If the medium is too basic, the initial protonation of the carbonyl fails, reducing electrophilicity. Thus, optimum pH (about 3–5) is crucial—a favourite concept for JEE Main application-type questions.
Summary: Typical Reactions and Application Table
| Aldehyde/Ketone | Reagent/Derivative | Product Name | Typical Use |
|---|---|---|---|
| R–CHO/R2C=O | NH3 | Imine (Schiff base) | Synthesis, ID |
| R–CHO/R2C=O | NH2OH | Oxime | Qualitative ID |
| R–CHO/R2C=O | 2,4-DNPH | Hydrazone | Screening test |
| R–CHO/R2C=O | Semicarbazide | Semicarbazone | Purification |
Remember, all these nucleophilic addition reactions require maintenance of correct pH conditions and often use gentle heating to push equilibrium towards completion. In competitive exams, questions may present a carbonyl and a derivative, asking for either the product, mechanism step order, or pH rationale.
The applications of these derivatives in identification and characterization of aldehydes and ketones are vital in both laboratory settings and JEE question patterns. For instance, the bright yellow precipitate given by 2,4-dinitrophenylhydrazine confirms a carbonyl compound, and determination of a melting point of a derivative often establishes the identity of the parent molecule.
Key Points: Optimal pH and Practical Traps
- Optimum pH (3–5) is essential—too acidic forms ammonium salts, too basic impairs carbonyl activation.
- Only primary and secondary amines (not tertiary) form imines or related derivatives (Schiff bases, oximes, hydrazones, etc.).
- Water elimination is needed to drive the reaction to completion (sometimes achieved by azeotropic distillation or trapping water).
- Carbonyl compounds with no α-hydrogens or hindered access may react slowly or give poor yields.
- Products are generally crystalline, aiding purification by recrystallization and confirming structure via melting point.
Mechanism Example: Addition to Acetone Using DNPH
Consider acetone reacting with 2,4-dinitrophenylhydrazine under mildly acidic conditions:
- Protonation of C=O increases carbon’s electrophilicity.
- DNPH (lone pair on N) attacks C=O carbon, forming a tetrahedral intermediate.
- Proton transfers yield an addition product which loses water, yielding the hydrazone (orange solid).
This sequence is archetypal in JEE for illustrated mechanistic MCQs and for product identification, where the appearance or isolation of a colourful solid means the reaction succeeded.
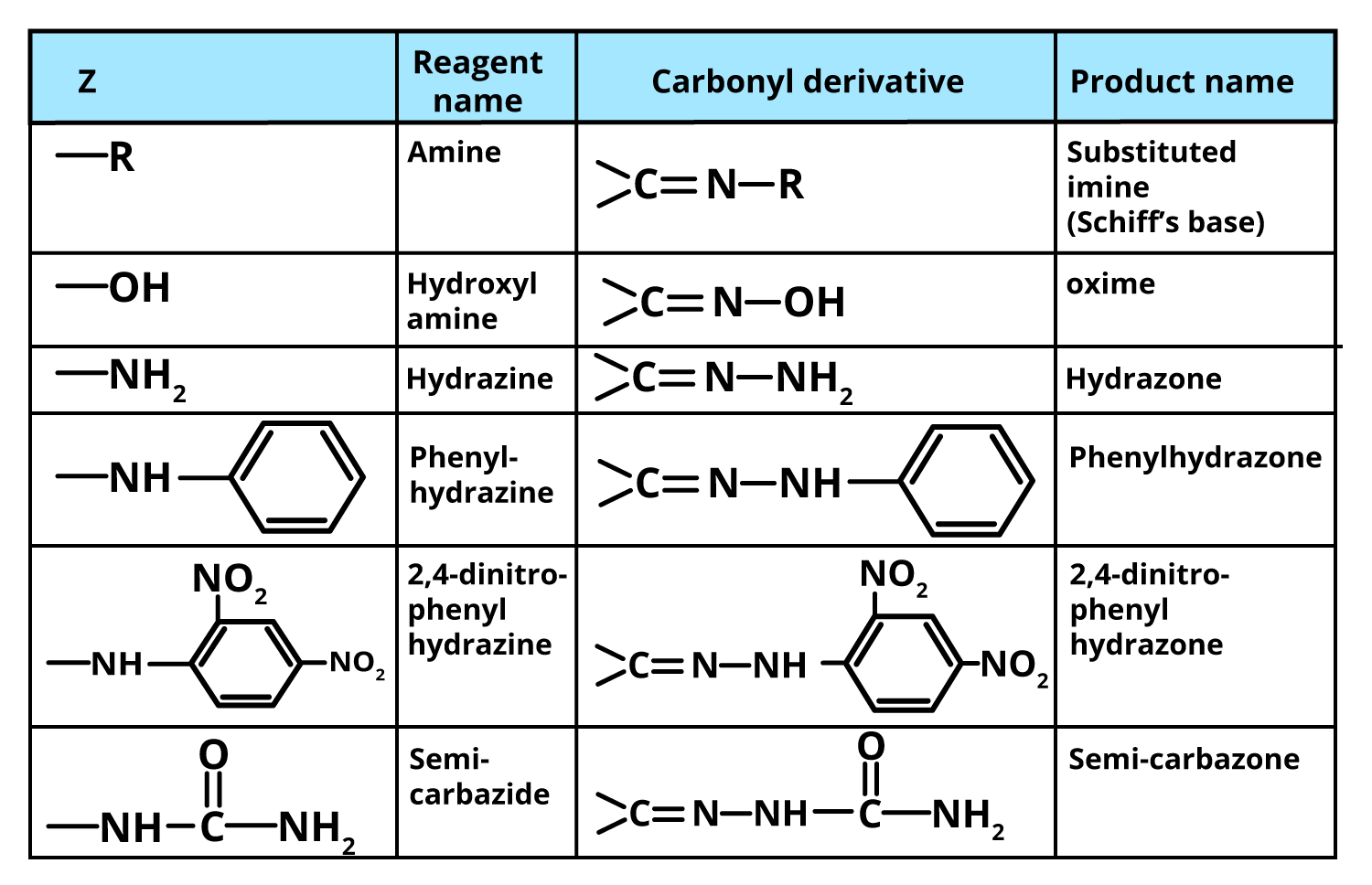
A complete revision should include knowing which derivative gives what product; for example, oximes from hydroxylamine, semicarbazones from semicarbazide, and hydrazones from hydrazine or DNPH. Also, note the final products can be hydrolysed back to the original carbonyl, confirming their diagnostic utility.
Quick-Revision Checklist for JEE
- Addition of Ammonia and Ammonia Derivatives = nucleophilic addition to C=O; mechanism involves protonation, nucleophilic attack, dehydration.
- Correct pH is always required for reaction to proceed and avoid non-nucleophilic ammonium salts.
- Key derivatives: hydrazine, semicarbazide, hydroxylamine, DNPH, phenylhydrazine.
- Schiff bases, oximes, hydrazones, semicarbazones: know structure and application for purification/identification.
- No imine formation with tertiary amines; mechanism may appear as assertion-reasoning or MCQ format.
- For application: melting points of derivatives help identify unknowns; use water removal or mild heat to improve yields.
- Practice with JEE mock tests and important questions for speed and accuracy in exam conditions.
For further mastery, review the nucleophilic addition reaction mechanisms in the Organic Compounds Containing Oxygen module and test your conceptual clarity through dedicated mock papers. Vedantu’s Chemistry coverage for JEE Main remains tightly focused on current syllabus and the latest NCERT guidelines to optimize your results.
In conclusion, mastery of the Addition of Ammonia and Ammonia Derivatives is indispensable for organic analysis and exam performance. A systematic, mechanism-led approach—backed by practice, optimum pH maintenance, and knowledge of each derivative’s unique application—will help secure marks in competitive chemistry. Review, revise, and practise with Vedantu’s mock tests to ensure full topic confidence for JEE Main.
FAQs on Addition of Ammonia and Ammonia Derivatives: Complete Guide for JEE Main
1. What is the mechanism of addition of ammonia and its derivatives to carbonyl compounds?
The mechanism of addition of ammonia and its derivatives to carbonyl compounds like aldehydes and ketones is a nucleophilic addition mechanism. The ammonia or its derivatives attack the carbonyl carbon, followed by loss of water, forming imines or similar products. Key steps include:
- Nucleophilic attack by ammonia or derivative on carbonyl carbon
- Formation of a tetrahedral intermediate
- Proton transfers facilitated by optimum pH
- Elimination of water molecule, yielding the final imine (Schiff base) or derivative formation
2. Why is optimum pH necessary for the addition of ammonia derivatives?
Optimum pH is essential during addition of ammonia derivatives to carbonyl compounds to ensure efficient reaction and product formation. If pH is too low or too high, the reaction yield decreases due to side reactions or reduced nucleophilicity. Reasons include:
- Prevention of over-protonation of ammonia/amine groups (which would make them less nucleophilic)
- Avoidance of reaction mixture becoming too basic, which could lead to unwanted side products
- Ensuring formation and stability of desired intermediates and final products
3. What are some common derivatives of ammonia used in organic reactions?
Common ammonia derivatives used in organic chemistry for addition to carbonyl compounds include:
- Primary amines (RNH2)
- Hydroxylamine (NH2OH)
- Hydrazine (NH2NH2)
- Semicarbazide (NH2NHCONH2)
These derivatives participate in similar mechanisms and lead to products like Schiff bases, oximes, hydrazones, and semicarbazones.
4. How is the reaction different for ketones and aldehydes?
Aldehydes generally react faster than ketones in the addition of ammonia and its derivatives because aldehydes are less sterically hindered and more electrophilic. Differences include:
- Aldehydes typically give higher yields and react more readily
- Ketones may require more rigorous conditions due to increased hindrance
- Both form similar imine-type products but at different rates
5. What notes or formulas should I remember for addition of ammonia and derivatives for JEE Main?
Key notes and formulas for JEE Main regarding addition of ammonia and derivatives include:
- General equation: R-CO-R' + NH3/Derivative → Imine/Schiff base + H2O
- Mechanism steps: Nucleophilic attack, intermediate formation, water elimination
- Optimum pH needed (around neutral)
- Examples: formation of oximes, hydrazones, semicarbazones
Quick revision of reaction conditions and product structures is essential for exams.
6. What is the purpose of adding ammonia or ammonia derivatives to carbonyl compounds?
The purpose is to convert carbonyl compounds into more stable derivatives for identification, purification, or analytical purposes in organic chemistry. This reaction:
- Helps in detecting and characterising aldehydes and ketones
- Forms products like imines, oximes, and hydrazones
- Is used in qualitative analysis and separation processes
- Is very important for JEE exam application-based questions
7. Can tertiary amines act as ammonia derivatives in these reactions?
Tertiary amines cannot participate effectively in the addition to carbonyl compounds because they lack a hydrogen atom on the nitrogen, which is needed for imine formation. Key points:
- Tertiary amines (R3N) do not form imines, oximes, or hydrazones
- Primary and secondary amines (with at least one N-H bond) are required
- For exam, remember that tertiary amines are not used for this reaction type
8. Does the addition of ammonia to a carbonyl compound always form imines?
No, the addition does not always result in imine formation. The product depends on the specific ammonia derivative used:
- Ammonia or primary amines form imines (Schiff bases)
- Hydroxylamine forms oximes
- Hydrazine forms hydrazones
- Semicarbazide forms semicarbazones
- Product structure is determined by the starting ammonia derivative
9. What happens if the reaction pH is too low or too high during addition of ammonia derivatives?
If pH is too low (acidic) or too high (basic), the addition reaction may give poor yield or fail. Effects include:
- In acidic pH, ammonia/amine gets protonated and loses nucleophilicity
- In basic pH, carbonyl groups become less electrophilic, slowing the reaction
- Optimum neutral pH is best for smooth reaction and product formation
- For exams, cite the importance of correct pH control in such reactions
10. Are there any exceptions to which carbonyl compounds undergo addition with ammonia derivatives?
Most aldehydes and ketones undergo addition with ammonia and its derivatives. However:
- Some highly hindered or deactivated ketones may react very slowly or not at all
- Compounds lacking an active carbonyl group (like carboxylic acids, esters) do not form imines in this reaction
- Aromatic ketones may need more severe conditions
- Always check the presence of a free carbonyl group for this mechanism to work
11. Explain the mechanism of nucleophilic addition of ammonia derivatives to carbonyl compounds.
The nucleophilic addition mechanism involves several steps:
- Step 1: Nucleophilic attack of ammonia derivative on the carbonyl carbon
- Step 2: Formation of a tetrahedral intermediate
- Step 3: Proton transfers within the intermediate
- Step 4: Elimination of water from the intermediate
- Step 5: Formation of an imine or related product (e.g., oxime, hydrazone)
This sequence is key for producing organic derivatives from aldehydes and ketones, tested often in JEE exams.
12. What are the applications of addition of ammonia and its derivatives in organic chemistry?
This reaction is widely applied in qualitative organic analysis and structure determination. Applications include:
- Formation of characteristic derivatives for identification of aldehydes and ketones
- Purification and separation of carbonyl compounds
- Use in synthetic organic chemistry for producing intermediates
- Frequently featured in JEE and NEET exam application-based questions























