
Bond length between carbon-carbon in ethylene molecule is
A) 1.54 \[\mathop A\limits^0 \]
B) 1.35 \[\mathop A\limits^0 \]
C) 1.19 \[\mathop A\limits^0 \]
D) 2.4 \[\mathop A\limits^0 \]
Answer
232.8k+ views
Hint: When the two atoms are bonded, the average distance between their nuclei is called the bond length. There is a relation between bond length with bond strength, bond order, and dissociation energy of the bond.
Complete Step by Step Answer:
Let's understand the ethylene molecule in detail. The molecule of ethylene is made of two carbons and one double bond. The ethene formula is as follows: \[{{\rm{H}}_{\rm{2}}}{\rm{C}} = {\rm{C}}{{\rm{H}}_{\rm{2}}}\].
The VSEPR theory predicts the spatial positions of C and H atoms of the ethene and also the bond angle. Each atom of carbon is surrounded by three other atoms in the ethylene molecule, therefore, the geometry of the molecule is trigonal planar and the bond angle is of \[120^\circ \] .
The following diagram shows the molecule of ethylene.
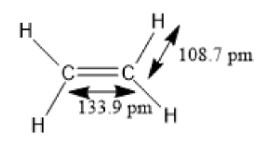
Image: Ethylene molecule
So, we see that the Carbon-Carbon bond in the molecule of ethylene has a bond length of 133.9 or 134 pm or 1.34 \[\mathop A\limits^0 \] and the carbon-hydrogen bond has a bond length of 108.7 pm or 1.09 \[\mathop A\limits^0 \] .
Hence, option B is right which is close to 1.34 \[\mathop A\limits^0 \].
Note: It is to be noted that, there is the inverse relation between bond length and bond order that is, Bond length\[\alpha \dfrac{{\rm{1}}}{{{\rm{Bond}}\,{\rm{order}}}}\] . Therefore, the bond length of ethane is more than ethylene and the bond length of ethylene is more than ethyne. The bond lengths of a single bond, double bond, and triple bond are 154 pm, 134 pm, and 120 pm respectively.
Complete Step by Step Answer:
Let's understand the ethylene molecule in detail. The molecule of ethylene is made of two carbons and one double bond. The ethene formula is as follows: \[{{\rm{H}}_{\rm{2}}}{\rm{C}} = {\rm{C}}{{\rm{H}}_{\rm{2}}}\].
The VSEPR theory predicts the spatial positions of C and H atoms of the ethene and also the bond angle. Each atom of carbon is surrounded by three other atoms in the ethylene molecule, therefore, the geometry of the molecule is trigonal planar and the bond angle is of \[120^\circ \] .
The following diagram shows the molecule of ethylene.

Image: Ethylene molecule
So, we see that the Carbon-Carbon bond in the molecule of ethylene has a bond length of 133.9 or 134 pm or 1.34 \[\mathop A\limits^0 \] and the carbon-hydrogen bond has a bond length of 108.7 pm or 1.09 \[\mathop A\limits^0 \] .
Hence, option B is right which is close to 1.34 \[\mathop A\limits^0 \].
Note: It is to be noted that, there is the inverse relation between bond length and bond order that is, Bond length\[\alpha \dfrac{{\rm{1}}}{{{\rm{Bond}}\,{\rm{order}}}}\] . Therefore, the bond length of ethane is more than ethylene and the bond length of ethylene is more than ethyne. The bond lengths of a single bond, double bond, and triple bond are 154 pm, 134 pm, and 120 pm respectively.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




