
Aniline on treatment with excess of bromine water gives:
A. Aniline Bromide
B. o-bromoaniline
C. p-bromoaniline
D. Benzene
Answer
233.1k+ views
Hint: Aniline acts as a strong base in acidic solution. It can donate its electron pair. Aniline has a basic amine or $NH_2$ group attached with benzene ring. The amine group is a electron donating group or $+I$ effect group.
Formula Used:
Complete step-by-step answer:In aniline the amine group attached to benzene ring is a strong electron donating group or $+I$ effect group. It is a ortho-para detecting group that means it direct the incoming electrophile to attack at ortho or para position of benzene ring.
Water is a polar solvent which activates the amine group of aniline so much that on treatment with bromine water the electrophile or the bromine atom attacks the two ortho and one para positions at a time and gives $2,4,6-tribromoaniline$ as a major product.
The bromination reaction of aniline in presence of excess bromine water is given as follows-
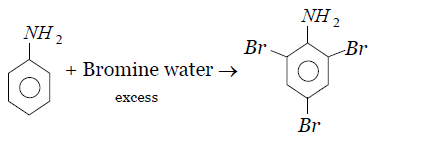
In this reaction aniline reacts with excess bromine water to produce $2,4,6-tribromoaniline$ as a major product with the minor product three moles of hydrogen bromide.
Option ‘ ’ is correct
Additional Information:
Note: Bromination reaction is an aromatic electrophilic substitution reaction where an electrophile attacks a benzene ring. If any electron donating group is connected with the benzene ring then reactivity of the benzene ring increases. Again if any electron withdrawing group is connected with the benzene ring then reactivity of the benzene ring decreases.
Formula Used:
Complete step-by-step answer:In aniline the amine group attached to benzene ring is a strong electron donating group or $+I$ effect group. It is a ortho-para detecting group that means it direct the incoming electrophile to attack at ortho or para position of benzene ring.
Water is a polar solvent which activates the amine group of aniline so much that on treatment with bromine water the electrophile or the bromine atom attacks the two ortho and one para positions at a time and gives $2,4,6-tribromoaniline$ as a major product.
The bromination reaction of aniline in presence of excess bromine water is given as follows-
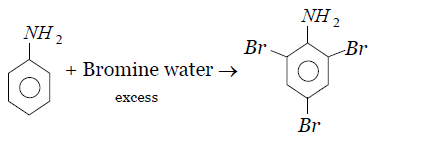
In this reaction aniline reacts with excess bromine water to produce $2,4,6-tribromoaniline$ as a major product with the minor product three moles of hydrogen bromide.
Option ‘ ’ is correct
Additional Information:
Note: Bromination reaction is an aromatic electrophilic substitution reaction where an electrophile attacks a benzene ring. If any electron donating group is connected with the benzene ring then reactivity of the benzene ring increases. Again if any electron withdrawing group is connected with the benzene ring then reactivity of the benzene ring decreases.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




