
An unknown aldehyde ′A′ on reacting with alkali gives a β- hydroxy aldehyde, which loses water to form an unsaturated aldehyde, but-2-enal. Another aldehyde ′B′ undergoes a disproportionation reaction in the presence of conc. alkali to form products C and D.C is aryl alcohol with the formula\[{C_7}{H_8}O\]. Name the product when ′B’ reacts with zinc amalgam and hydrochloric acid.
Answer
233.1k+ views
Hint: Aldehydes react with dilute alkalis to form β-hydroxy aldehydes which then lose water to form unsaturated aldehydes. This reaction is called Aldol Condensation. In the presence of concentrated alkalis, aldehydes with no α-hydrogen atoms undergo disproportionation. This reaction is called Cannizzaro Reaction.
Complete Step by Step Solution:
The hydrogen atom present at alpha-position to the carbonyl group in an aldehyde is acidic due to the strong electron-withdrawing effect of the carbonyl and resonance stabilisation of the conjugate base as shown below:
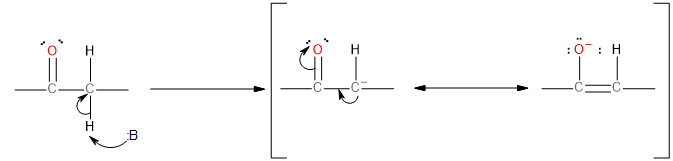
Image: Acidity of Alpha Hydrogen
Aldehydes having at least one α-hydrogen atom undergo a reaction in the presence of dilute alkali to form β-hydroxy aldehydes. This reaction is called Aldol Reaction. β-hydroxy aldehydes (also known as aldols) lose water readily to form α,β-unsaturated aldehydes. This reaction is called Aldol Condensation.
Here, we have an unknown aldehyde ‘A’ which forms a β-hydroxy aldehyde in reaction with alkali. The β-hydroxy aldehyde then loses a water molecule to form but-2-enal which is an α,β-unsaturated aldehyde. Thus, we can identify the reaction as an aldol condensation.
Since the α,β-unsaturated aldehyde is given to be but-2-enal, we can work our way backwards to identify the β-hydroxy aldehyde. The β-hydroxy aldehyde will have the same number of carbon atoms in its parent chain as but-2-enal (i.e., 4 carbon atoms), except, it will have a hydroxyl functional group (\[ - OH\]) at the β-position as shown below.
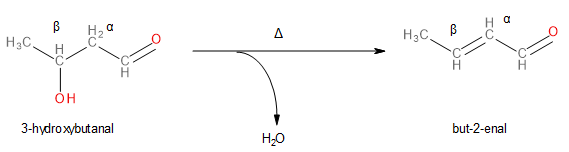
Image: Dehydration of 3-Hydroxybutanal to form But-2-enal.
3-hydroxybutanal is the product of an aldol reaction. Working our way backwards similarly, we can figure out that the unknown aldehyde ‘A’ is ethanal (or acetaldehyde) because ethanal is the only aldehyde that can undergo aldol reaction to give 3-hydroxybutanal.
Image: Aldol condensation of Ethanal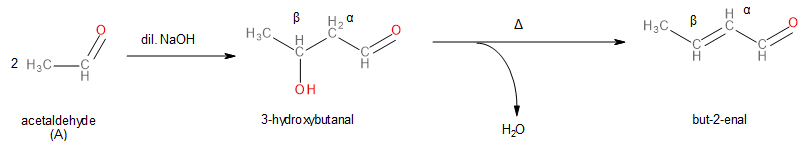
Aldehydes without any α-hydrogen atoms undergo a disproportionation reaction in the presence of concentrated alkali. In this reaction, one molecule of aldehyde is reduced to alcohol while another molecule of aldehyde is oxidised to a carboxylic acid. This reaction is called the Cannizzaro reaction.
According to the question, aldehyde ‘B’ disproportionates into an aryl alcohol C with the chemical formula \[{C_7}{H_8}O\] and D which must be a carboxylic acid. Since C has an aryl group present in it, we can safely say that aldehyde ‘B’ must also be an aryl aldehyde. The aryl alcohol that has the chemical formula\[{C_7}{H_8}O\] is most likely benzyl alcohol. The aldehyde that underwent reduction to form benzyl alcohol must be benzaldehyde which is the unknown aldehyde ‘B’.
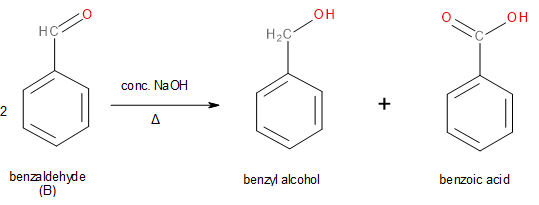
Image: Cannizaro reaction of Benzaldehyde
When aldehyde ‘B’ (benzaldehyde) reacts with zinc amalgam (\[Zn - Hg\]) and hydrochloric acid (\[HCl\]), its carbonyl group is reduced to \[C{H_2}\]group. This reduction is called Clemmensen reduction. Thus, benzaldehyde will get reduced to toluene.
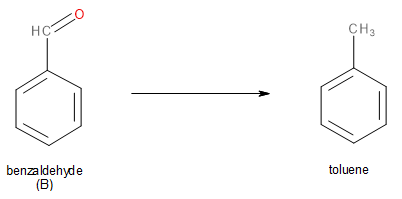
Image: Clemmensen Reduction of Benzaldehyde to Toluene.
The product formed is toluene.
Note: Aldol reaction occurs in aldehydes with at least one α-hydrogen whereas the Cannizzaro reaction occurs in aldehydes with no α-hydrogen. Another thing to keep in mind is that the product of the aldol reaction (the β-hydroxy aldehyde) will have twice the number of carbon atoms in its parent chain as the reactant aldehyde.
Complete Step by Step Solution:
The hydrogen atom present at alpha-position to the carbonyl group in an aldehyde is acidic due to the strong electron-withdrawing effect of the carbonyl and resonance stabilisation of the conjugate base as shown below:

Image: Acidity of Alpha Hydrogen
Aldehydes having at least one α-hydrogen atom undergo a reaction in the presence of dilute alkali to form β-hydroxy aldehydes. This reaction is called Aldol Reaction. β-hydroxy aldehydes (also known as aldols) lose water readily to form α,β-unsaturated aldehydes. This reaction is called Aldol Condensation.
Here, we have an unknown aldehyde ‘A’ which forms a β-hydroxy aldehyde in reaction with alkali. The β-hydroxy aldehyde then loses a water molecule to form but-2-enal which is an α,β-unsaturated aldehyde. Thus, we can identify the reaction as an aldol condensation.
Since the α,β-unsaturated aldehyde is given to be but-2-enal, we can work our way backwards to identify the β-hydroxy aldehyde. The β-hydroxy aldehyde will have the same number of carbon atoms in its parent chain as but-2-enal (i.e., 4 carbon atoms), except, it will have a hydroxyl functional group (\[ - OH\]) at the β-position as shown below.

Image: Dehydration of 3-Hydroxybutanal to form But-2-enal.
3-hydroxybutanal is the product of an aldol reaction. Working our way backwards similarly, we can figure out that the unknown aldehyde ‘A’ is ethanal (or acetaldehyde) because ethanal is the only aldehyde that can undergo aldol reaction to give 3-hydroxybutanal.
Image: Aldol condensation of Ethanal

Aldehydes without any α-hydrogen atoms undergo a disproportionation reaction in the presence of concentrated alkali. In this reaction, one molecule of aldehyde is reduced to alcohol while another molecule of aldehyde is oxidised to a carboxylic acid. This reaction is called the Cannizzaro reaction.
According to the question, aldehyde ‘B’ disproportionates into an aryl alcohol C with the chemical formula \[{C_7}{H_8}O\] and D which must be a carboxylic acid. Since C has an aryl group present in it, we can safely say that aldehyde ‘B’ must also be an aryl aldehyde. The aryl alcohol that has the chemical formula\[{C_7}{H_8}O\] is most likely benzyl alcohol. The aldehyde that underwent reduction to form benzyl alcohol must be benzaldehyde which is the unknown aldehyde ‘B’.

Image: Cannizaro reaction of Benzaldehyde
When aldehyde ‘B’ (benzaldehyde) reacts with zinc amalgam (\[Zn - Hg\]) and hydrochloric acid (\[HCl\]), its carbonyl group is reduced to \[C{H_2}\]group. This reduction is called Clemmensen reduction. Thus, benzaldehyde will get reduced to toluene.

Image: Clemmensen Reduction of Benzaldehyde to Toluene.
The product formed is toluene.
Note: Aldol reaction occurs in aldehydes with at least one α-hydrogen whereas the Cannizzaro reaction occurs in aldehydes with no α-hydrogen. Another thing to keep in mind is that the product of the aldol reaction (the β-hydroxy aldehyde) will have twice the number of carbon atoms in its parent chain as the reactant aldehyde.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




