
An ideal gas is taken from the same initial pressure P1 to the same final pressure P2 by three different processes. If it is known that point 1 corresponds to a reversible adiabatic and point 2 corresponds to a single-stage adiabatic then:
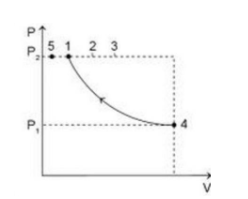
A)Point 3 may be a two-stage adiabatic process
B)The avg. K.E. of the gas is maximum at point 1
C)Work done by surroundings in reaching points 1 or 2 will be positive.
D)The points 3 and 4 may lie along the process having ${\text{P}}{{\text{V}}^2} = $constant
Answer
233.1k+ views
Hint: To answer this question, you should recall the concept of an adiabatic expansion of a gas. We know that adiabatic expansion is defined as an ideal behaviour for a closed system, in which the pressure is constant and the temperature is decreasing. Use the formula of work done to determine the work done by each process.
Complete Step by step Answer:
We know that during the adiabatic process, no heat enters or leaves the system. Hence during compression, the temperature will increase. The work done in the reversible adiabatic process is given by the expression: \[{{\text{w}}_{{\text{rev}}}} = \dfrac{{{\text{nR}} \times \left( {{{\text{T}}_{\text{2}}}{\text{ - }}{{\text{T}}_{\text{1}}}} \right)}}{{\gamma - 1}}\;\]
We can see from the graph plotted that \[{{\text{T}}_{\text{2}}}{\text{ > }}{{\text{T}}_{\text{1}}}_{\text{\;}}\] for the process in which the gas is taken from point 4 to point 1, the work done will be positive. This means that work is being on the system.
Similarly, the work done will be positive when the system is taken from point 4 to point 2.
Hence option C is correct.
We are aware that the ideal gas equation is given by: \[{\text{PV = nRT}}\]. Rearranging this equation, we get, $\dfrac{{{\text{PV}}}}{{\text{T}}}$=constant. In the process when gas is taken from 4 to 3, as the volume decreases, the temperature increases. Comparing this with the ideal gas equation, we can say that \[{{\text{T}}_{\text{1}}}{\text{ }}\alpha {\text{ V}}\]. Hence, it may be possible that \[{\text{P}}{{\text{V}}^{\text{2}}}\] =constant. Thus, option D is correct.
Therefore, we can conclude that the correct answer to this question is option C and option D.
Note: We can confuse between different types of reactions. Make sure to remember the difference between isobaric, isochoric, isothermal and adiabatic processes. An isobaric process is one where the pressure of the system (often a gas) stays constant. An isochoric process is one where the volume of the system stays constant. An adiabatic process is a thermodynamic process in which no heat is exchanged between the system and the surrounding.
Complete Step by step Answer:
We know that during the adiabatic process, no heat enters or leaves the system. Hence during compression, the temperature will increase. The work done in the reversible adiabatic process is given by the expression: \[{{\text{w}}_{{\text{rev}}}} = \dfrac{{{\text{nR}} \times \left( {{{\text{T}}_{\text{2}}}{\text{ - }}{{\text{T}}_{\text{1}}}} \right)}}{{\gamma - 1}}\;\]
We can see from the graph plotted that \[{{\text{T}}_{\text{2}}}{\text{ > }}{{\text{T}}_{\text{1}}}_{\text{\;}}\] for the process in which the gas is taken from point 4 to point 1, the work done will be positive. This means that work is being on the system.
Similarly, the work done will be positive when the system is taken from point 4 to point 2.
Hence option C is correct.
We are aware that the ideal gas equation is given by: \[{\text{PV = nRT}}\]. Rearranging this equation, we get, $\dfrac{{{\text{PV}}}}{{\text{T}}}$=constant. In the process when gas is taken from 4 to 3, as the volume decreases, the temperature increases. Comparing this with the ideal gas equation, we can say that \[{{\text{T}}_{\text{1}}}{\text{ }}\alpha {\text{ V}}\]. Hence, it may be possible that \[{\text{P}}{{\text{V}}^{\text{2}}}\] =constant. Thus, option D is correct.
Therefore, we can conclude that the correct answer to this question is option C and option D.
Note: We can confuse between different types of reactions. Make sure to remember the difference between isobaric, isochoric, isothermal and adiabatic processes. An isobaric process is one where the pressure of the system (often a gas) stays constant. An isochoric process is one where the volume of the system stays constant. An adiabatic process is a thermodynamic process in which no heat is exchanged between the system and the surrounding.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




