
Among the following which one does not act as an intermediate in Hofmann rearrangement?
A. RNCO
B. RCON
C. RCONHBr
D. RNC
Answer
233.1k+ views
Hint: Hofmann rearrangement also called Hofmann degradation is a rearrangement reaction of primary amides with bromine and aqueous or alcoholic potassium hydroxide to give primary amines. Overall, in the end, the carbonyl group get removed from the product.
Complete Step by Step Solution:
A lot of intermediates can be found in this reaction because of a lot of steps present in the reaction. After step-by-step solving the reaction, all three above intermediates formed in the reaction except option D i.e., RNC. The reaction mechanism is shown below:
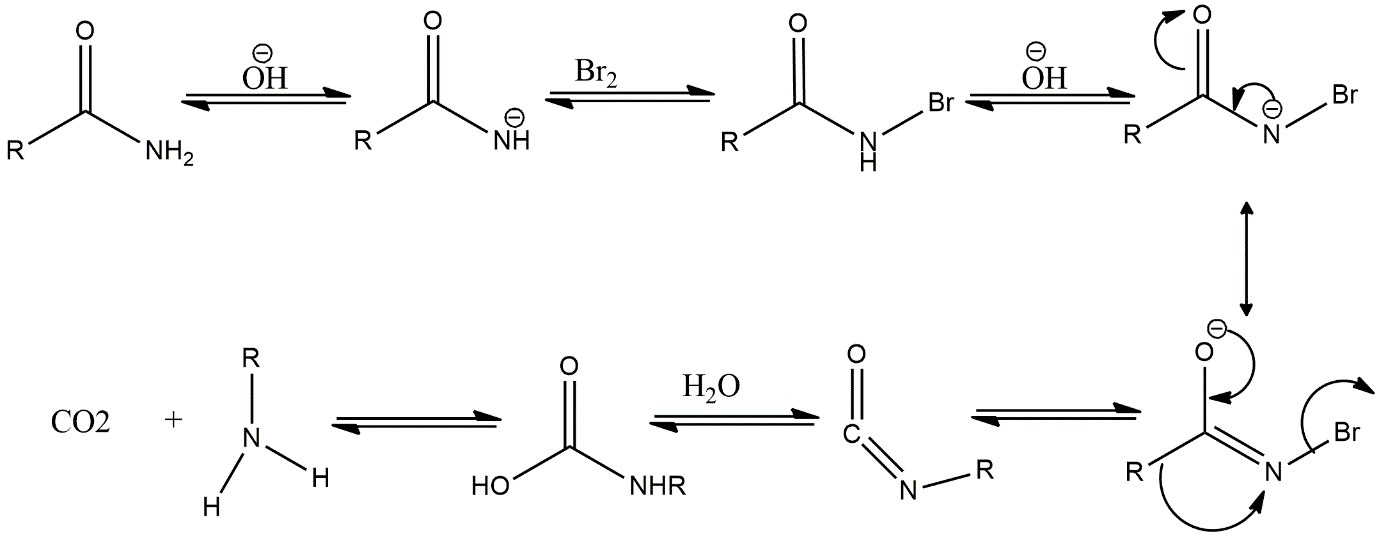
Image:Mechanism of Hoffmann rearrangement
At first hydroxyl ion of the base \[KOH\]accepts \[{H^ + }\]from \[N\]of amide functional group to form an \[RCON\]intermediate, then \[B{r^ + }\]of \[B{r_2}\]attacks on \[{N^ - }\]of intermediate to form\[RCONHBr\]. Then again, the base accepts another \[{H^ + }\]of the amide functional group. Now, the \[R\]group migrates to Nitrogen and \[Br\]ion leaves as it is a leaving group and gives an \[RNCO\]intermediate. After that, proton and hydroxyl ions of water get added to the intermediate and give carbamic acid which gives amine as a final product with the loss of carbon dioxide gas.
So, the option D is correct.
Additional Information:The carbon dioxide gas released in the last step is the driving force of the reaction as this increases the entropy of the reaction, due to which the reaction becomes spontaneous.
Note: Hofmann rearrangement is an organic reaction of primary amides and not the Hofmann elimination reaction. Hofmann elimination is the elimination reaction to form an alkene product.
Complete Step by Step Solution:
A lot of intermediates can be found in this reaction because of a lot of steps present in the reaction. After step-by-step solving the reaction, all three above intermediates formed in the reaction except option D i.e., RNC. The reaction mechanism is shown below:

Image:Mechanism of Hoffmann rearrangement
At first hydroxyl ion of the base \[KOH\]accepts \[{H^ + }\]from \[N\]of amide functional group to form an \[RCON\]intermediate, then \[B{r^ + }\]of \[B{r_2}\]attacks on \[{N^ - }\]of intermediate to form\[RCONHBr\]. Then again, the base accepts another \[{H^ + }\]of the amide functional group. Now, the \[R\]group migrates to Nitrogen and \[Br\]ion leaves as it is a leaving group and gives an \[RNCO\]intermediate. After that, proton and hydroxyl ions of water get added to the intermediate and give carbamic acid which gives amine as a final product with the loss of carbon dioxide gas.
So, the option D is correct.
Additional Information:The carbon dioxide gas released in the last step is the driving force of the reaction as this increases the entropy of the reaction, due to which the reaction becomes spontaneous.
Note: Hofmann rearrangement is an organic reaction of primary amides and not the Hofmann elimination reaction. Hofmann elimination is the elimination reaction to form an alkene product.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




