
Alkene can be prepared from alkyl halide by the following reagent \[{\rm{R}} - {\rm{X}} + N{u^ - } \to {\rm{Alkene}} + NuH\]
A) Alc. KOH + Heat
B) Aq KOH + Cold water
C) NaOH
D) LiOH
Answer
233.1k+ views
Hint: The reaction of an alkyl halide with potassium hydroxide of the alcoholic nature gives the elimination reaction. This reaction is also termed dehydrohalogenation reaction.
Complete Step by Step Solution:
Let's understand the dehydrohalogenation reaction in detail. In this reaction, an alkyl halide undergoes a reaction with a base whose nature is strong, that is, potassium hydroxide (alcoholic). In this reaction, OH, a strong base, attracts one proton from the beta carbon. This causes the release of one hydrogen atom and one atom of chlorine from the alkyl halide and the formation of alkene takes place. And this reaction is called \[\beta \] elimination because of the removal of a proton from the \[\beta \]carbon. A beta carbon indicates the carbon atom beside the carbon atom to which the halogen atom forms a bond.
For example, in the reaction of ethyl bromide with alcoholic KOH, the strong base, OH attracts a proton and the Br atom leaves the compound. This results in the formation of an unsaturated compound, that is, ethene or ethylene.
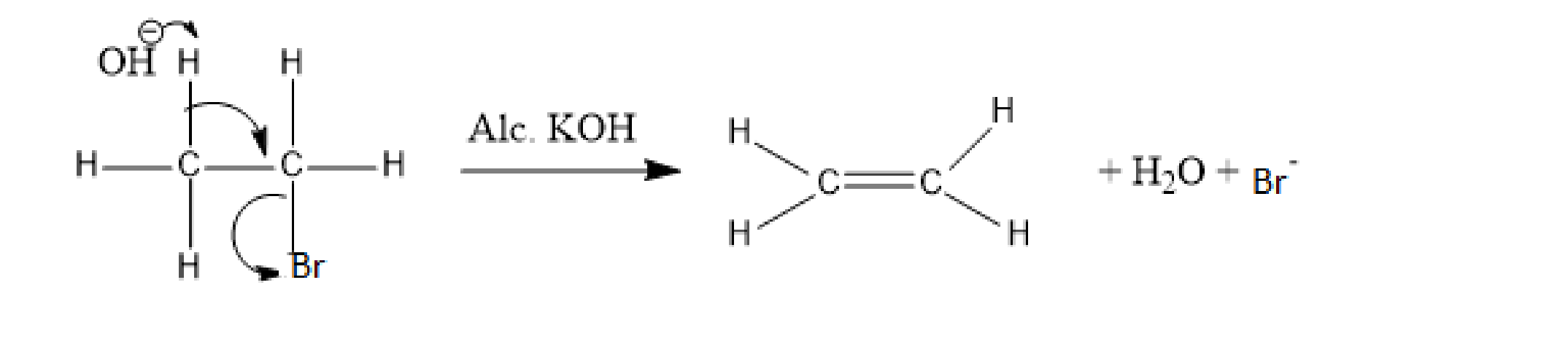
Image: Dehydrohalogenation reaction
Here, OH acts as a nucleophile.
Therefore, option A is right.
Note: It is the point of confusion among students about the reaction of an alkyl halide with alcoholic KOH and aqueous KOH. As stated above, a reaction with alcoholic KOH of a haloalkane gives an elimination reaction. And the reaction with aqueous KOH gives a substitution reaction, so the product obtained is alcohol.
Complete Step by Step Solution:
Let's understand the dehydrohalogenation reaction in detail. In this reaction, an alkyl halide undergoes a reaction with a base whose nature is strong, that is, potassium hydroxide (alcoholic). In this reaction, OH, a strong base, attracts one proton from the beta carbon. This causes the release of one hydrogen atom and one atom of chlorine from the alkyl halide and the formation of alkene takes place. And this reaction is called \[\beta \] elimination because of the removal of a proton from the \[\beta \]carbon. A beta carbon indicates the carbon atom beside the carbon atom to which the halogen atom forms a bond.
For example, in the reaction of ethyl bromide with alcoholic KOH, the strong base, OH attracts a proton and the Br atom leaves the compound. This results in the formation of an unsaturated compound, that is, ethene or ethylene.

Image: Dehydrohalogenation reaction
Here, OH acts as a nucleophile.
Therefore, option A is right.
Note: It is the point of confusion among students about the reaction of an alkyl halide with alcoholic KOH and aqueous KOH. As stated above, a reaction with alcoholic KOH of a haloalkane gives an elimination reaction. And the reaction with aqueous KOH gives a substitution reaction, so the product obtained is alcohol.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




