
A tin chloride Q undergoes the subsequent reaction (not balanced)
$Q+C{{l}^{-}}\text{ }\to \text{ }X$
$Q+\text{ }M{{e}_{3}}N\to \text{ }Y$
$Q+\text{ }CuC{{l}_{2}}\text{ }\to \text{ }Z+\text{ }CuCl$
X is a monoanion having pyramidal geometry. Both Y and Z are neutral compounds. Choose the right options (this question has multiple correct option)
(A) The central atom in X is $s{{p}^{3}}$ hybridized
(B) There is a dative bond in Y
(C) The oxidation number of the central atom in Z is +2
(D) The central atom in Z has one lone pair of electrons
Answer
232.8k+ views
Hint: As we all know the formula for tin chloride is $SnC{{l}_{2}}$ (where during this question performs some reactions). Monoanion is mentioned as an ion having one charge. Where pyramidal geometry is made by a polyhedron formed by connecting a polygonal base and some extent, called the apex.
Complete step by step answer: In the unified state, the metals are meant to exist as they are present in nature within their compounds. The reactive metals typically exist inside their compound appearance. In the cumulative state the metals are found as oxides, carbonates, sulphides, silicates, phosphates etc. within the planet's crust. Composite Q is $SnC{{l}_{2}}$.
The given reactions are
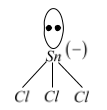
$SnC{{l}_{2}}+C{{l}^{-}}\to \underset{\left( X \right)}{\mathop{SnCl_{3}^{-}}}\,$
$SnC{{l}_{2}}+M{{e}_{3}}N\to \underset{\left( Y \right)}{\mathop{M{{e}_{3}}\overset{+}{\mathop{N}}\,-\overset{-}{\mathop{Sn}}\,C{{l}_{2}}}}\,$
$SnC{{l}_{2}}+2CuC{{l}_{2}}\to SnC{{l}_{4}}+\underset{\left( Z \right)}{\mathop{2CuCl}}\,$
Hybridisation of $Sn$ in $SnCl_{3}^{-}$is $s{{p}^{3}}$(X)
Trimethylamine forms a co-ordinate bond with $Sn$ in $SnCl_{2}^{{}}$(Y),
and in $SnCl_{4}^{{}}$ there's no lone pair on the central atom of $SnCl_{4}^{{}}$ (Z).
Hence, (Q), (X), (Y) and (Z) are $SnC{{l}_{2}}$, $SnCl_{3}^{-}$, $SnCl_{2}^{{}}$ and $SnCl_{4}^{{}}$ respectively.
Tin (II) chloride is a white crystalline solid with the formula $SnC{{l}_{2}}$ and is also known as stannous chloride. It forms a stable dihydrate, but aqueous solutions, especially if acidic, tend to undergo hydrolysis. $SnC{{l}_{2}}$ is commonly used as a reducing agent, and for tin-plating in electrolytic baths.
Note: Tin ($Sn$) may well be a bunch 14 elements, it show $s{{p}^{3}}$ hybridisation with two bond pairs between $s{{p}^{3}}-p$ orbital of two chlorine atoms and remaining two lone pairs are present. the shape of the molecule is V-shaped.
Complete step by step answer: In the unified state, the metals are meant to exist as they are present in nature within their compounds. The reactive metals typically exist inside their compound appearance. In the cumulative state the metals are found as oxides, carbonates, sulphides, silicates, phosphates etc. within the planet's crust. Composite Q is $SnC{{l}_{2}}$.
The given reactions are
$SnC{{l}_{2}}+C{{l}^{-}}\to \underset{\left( X \right)}{\mathop{SnCl_{3}^{-}}}\,$
$SnC{{l}_{2}}+M{{e}_{3}}N\to \underset{\left( Y \right)}{\mathop{M{{e}_{3}}\overset{+}{\mathop{N}}\,-\overset{-}{\mathop{Sn}}\,C{{l}_{2}}}}\,$
$SnC{{l}_{2}}+2CuC{{l}_{2}}\to SnC{{l}_{4}}+\underset{\left( Z \right)}{\mathop{2CuCl}}\,$
Hybridisation of $Sn$ in $SnCl_{3}^{-}$is $s{{p}^{3}}$(X)

Trimethylamine forms a co-ordinate bond with $Sn$ in $SnCl_{2}^{{}}$(Y),
and in $SnCl_{4}^{{}}$ there's no lone pair on the central atom of $SnCl_{4}^{{}}$ (Z).
Hence, (Q), (X), (Y) and (Z) are $SnC{{l}_{2}}$, $SnCl_{3}^{-}$, $SnCl_{2}^{{}}$ and $SnCl_{4}^{{}}$ respectively.
Tin (II) chloride is a white crystalline solid with the formula $SnC{{l}_{2}}$ and is also known as stannous chloride. It forms a stable dihydrate, but aqueous solutions, especially if acidic, tend to undergo hydrolysis. $SnC{{l}_{2}}$ is commonly used as a reducing agent, and for tin-plating in electrolytic baths.
Note: Tin ($Sn$) may well be a bunch 14 elements, it show $s{{p}^{3}}$ hybridisation with two bond pairs between $s{{p}^{3}}-p$ orbital of two chlorine atoms and remaining two lone pairs are present. the shape of the molecule is V-shaped.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




