
A capillary tube of radius r is dipped in a liquid of density $\rho $ and surface tension S. If the angle of contact is $\theta $, the pressure difference between the two surfaces in the beaker and the capillary is
A. $\dfrac{s}{r}\cos \theta $
B. $\dfrac{2s}{r}\cos \theta $
C. $\dfrac{s}{r\cos \theta }$
D. $\dfrac{2s}{r\cos \theta }$
Answer
233.1k+ views
Hint: The surface tension of a liquid is defined as the amount of energy or work necessary to raise the surface area of the liquid due to intermolecular forces in the particles in that liquid. In this case, we are given that the capillary tube is dipped in water and it is concave and the angle of contact is given as \[\theta \] and we are to determine the pressure difference. For that, we have to use the pressure difference formula and solve it accordingly to determine the desired answer.
Formula used:
Pressure difference can be calculated using
Pressure difference \[ = {\rm{hdg}}\]
Complete step by step solution:
The water meniscus inside a capillary tube is concave when it is dipped in water. The pressure close below the meniscus is \[\dfrac{{2S}}{R}\] less than the pressure just above it, where S is surface tension and R is the meniscus’s radius of curvature.
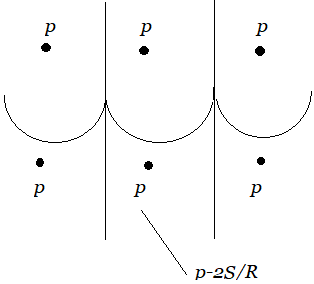
The pressure which exists on the surface is atmospheric pressure P. The pressure just below the plane surface of water outside the tube is also P, but that exists just below the meniscus inside the tube is $P-\dfrac{2S}{R}$.
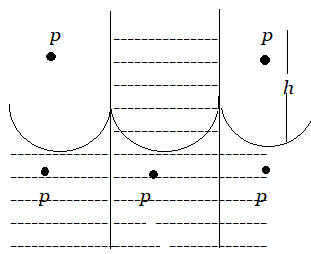
As a result, water begins to flow from outside the tube to compensate for the pressure deficiency \[\dfrac{{2S}}{R}\] below the meniscus. The water that is rising in the capillary stops at a specific height of h. The pressure of the water column of height h becomes equal to \[\dfrac{{2S}}{R}\] in this location.
\[P = h\rho g = \dfrac{{2S}}{R}\]
Where \[\rho \] is density
Then \[g\] is the gravity
If r is the radius of tube and \[\theta \] is angle of contact then
\[R = \dfrac{r}{{\cos \theta }}\]
Therefore, we can write the above as
\[P = h\rho g\]
On replacing the specific value, we get
\[P= \dfrac{{2S\cos \theta }}{r}\]
Thus, option B is the correct answer.
Note: Students should keep in mind that capillary rise will occur only if the adhesive forces are greater than the cohesive forces and also that water rises in the capillary tube due to the forces of cohesive force, adhesive force, and surface tension. And one should find it difficult to solve these types of problems because it involves trig functions and some formulas.
Formula used:
Pressure difference can be calculated using
Pressure difference \[ = {\rm{hdg}}\]
Complete step by step solution:
The water meniscus inside a capillary tube is concave when it is dipped in water. The pressure close below the meniscus is \[\dfrac{{2S}}{R}\] less than the pressure just above it, where S is surface tension and R is the meniscus’s radius of curvature.

The pressure which exists on the surface is atmospheric pressure P. The pressure just below the plane surface of water outside the tube is also P, but that exists just below the meniscus inside the tube is $P-\dfrac{2S}{R}$.

As a result, water begins to flow from outside the tube to compensate for the pressure deficiency \[\dfrac{{2S}}{R}\] below the meniscus. The water that is rising in the capillary stops at a specific height of h. The pressure of the water column of height h becomes equal to \[\dfrac{{2S}}{R}\] in this location.
\[P = h\rho g = \dfrac{{2S}}{R}\]
Where \[\rho \] is density
Then \[g\] is the gravity
If r is the radius of tube and \[\theta \] is angle of contact then
\[R = \dfrac{r}{{\cos \theta }}\]
Therefore, we can write the above as
\[P = h\rho g\]
On replacing the specific value, we get
\[P= \dfrac{{2S\cos \theta }}{r}\]
Thus, option B is the correct answer.
Note: Students should keep in mind that capillary rise will occur only if the adhesive forces are greater than the cohesive forces and also that water rises in the capillary tube due to the forces of cohesive force, adhesive force, and surface tension. And one should find it difficult to solve these types of problems because it involves trig functions and some formulas.
Recently Updated Pages
Dimensions of Charge: Dimensional Formula, Derivation, SI Units & Examples

How to Calculate Moment of Inertia: Step-by-Step Guide & Formulas

Circuit Switching vs Packet Switching: Key Differences Explained

Dimensions of Pressure in Physics: Formula, Derivation & SI Unit

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




