




Step-by-Step Birch Reduction Mechanism & Key Applications
The first chemist who gave birch reduction was “Carl Djerassi”. He is also called the “father of The Pill”. Birch reduction occurs in the presence of sodium or potassium or lithium metal dissolved in ammonia and alcohol. In this reduction, aromatic rings are reduced with sodium or potassium or lithium dissolved in liquid ammonia and in the presence of alcohol to give an unconjugated diene with the addition of two hydrogens at opposite sides. Liquid ammonia serves as a solvent.
Birch Reduction Mechanism
The accepted mechanism of birch reduction involves the following steps:
The metal transfers one electron to the aromatic ring to produce a resonance-stabilised anion radical.
Now, this anion radical accepts a proton from alcohol to form a second radical.
The addition of an electron from the metal to the above radical forms an anion which further takes a proton from alcohol to give the product.
Birch Reduction of Benzene and its Mechanism
When benzene reacts with sodium or potassium or lithium metal in the presence of alcohol, it forms 1,4-cyclohexadiene. This reaction is called birch reduction of benzene.
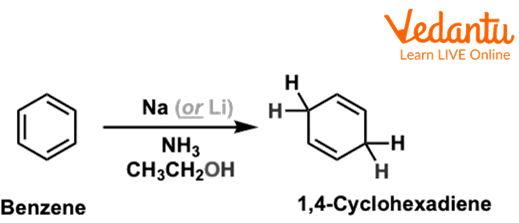
Birch reduction of Benzene
The above reaction shows the reduction of benzene, in which benzene reacts with Sodium in liquid ammonia and ethane and forms 1,4-cyclohexadiene.
The mechanism of birch reduction of benzene involves the following three steps:
In the first step, Sodium metal transfers one electron to the benzene ring to produce a resonance-stabilised radical anion.
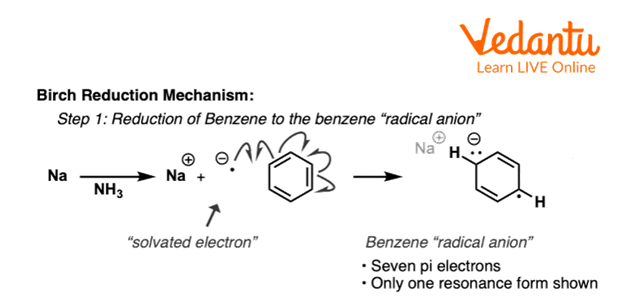
First Step of Birch Reduction of Benzene
The above reaction shows the first step of birch reduction of benzene in which benzene reacts with lithium and forms a benzene anion radical.
Now, this benzene anion radical accepts a proton from ethyl alcohol to form a second benzene radical.
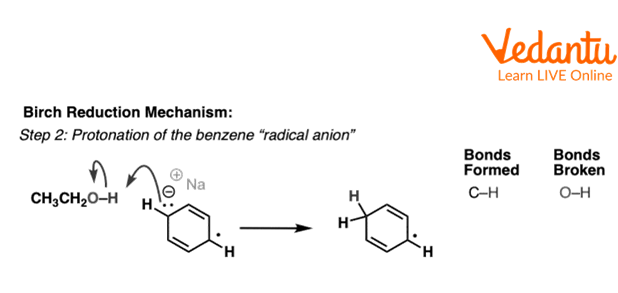
Second Step of Birch Reduction of Benzene
The above reaction shows the second step of birch reduction of benzene in which benzene anion radical accepts a proton from ethanol and forms benzene radical.
Now, this benzene radical takes an electron from metal and forms an anion which further takes a proton from ethyl alcohol and forms the final product 1,4-dihydrocyclohexadiene.
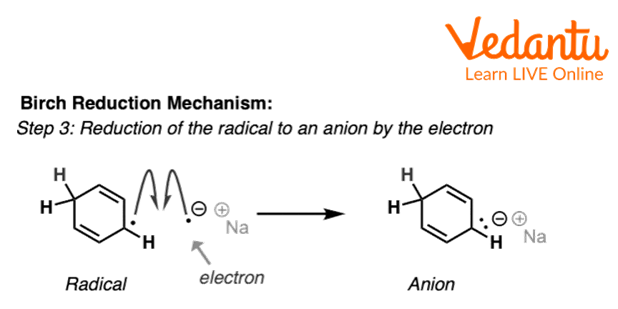
Third Step of Birch Reduction of Benzene
The above reaction shows the third step of birch reduction of benzene in which benzene radical reacts with sodium to form benzene radical anion which further accepts a proton from ethanol and forms the final product.
It is at this stage where the presence of an alcohol (e.g., ethanol or t-butanol) becomes necessary since NH3 is not a strong enough acid to protonate this anion. Protonation of this species, at the central carbon, results in the 1,4-cyclohexadiene.
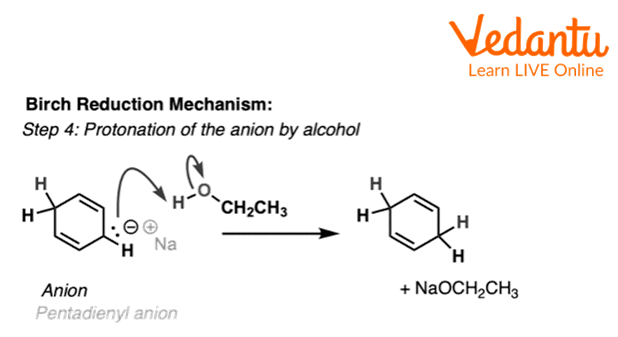
Fourth Step of Birch Reduction of Benzene
Birch Reduction of Alkyne and its Mechanism
Alkynes are also known to undergo birch reduction to form alkenes. Alkynes form trans-alkenes in birch reduction. The terminal alkynes do not show birch reduction because the alkyne proton is acidic enough to react with the dissolving metal to give the anion.
An example of birch reduction of the alkyne is given below:
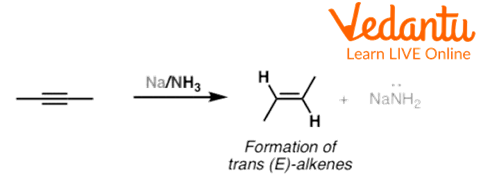
Birch Reduction of but-2-yne
The mechanism of birch reduction of alkyne involves the following three steps:
The first step in the reduction of alkyne involves the transfer of an electron from sodium metal to the triple bond and forms an anion radical.
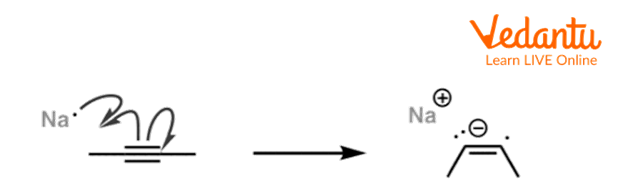
First step of Birch Reduction of but-2-yne
The above reaction is the first step of birch reduction of but-2-yne in which but-2-yne reacts with sodium metal and forms a radical anion.
In the second step, this anion accepts a proton from ammonia and forms a radical species.
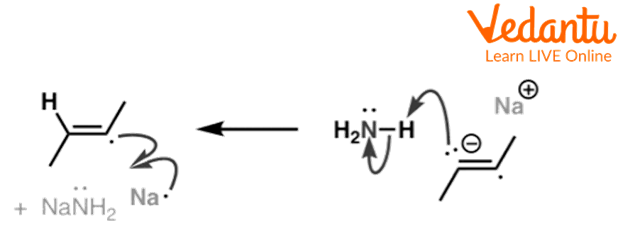
Second step of Birch Reduction of but-2-yne
The above reactions show the second step of reduction of but-2-yne in which a radical anion accepts a proton from ammonia and forms a radical.
In the last step, this radical species takes a proton from ammonia and forms a trans alkene.
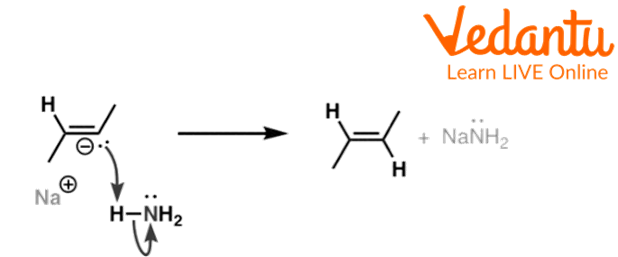
Third Step of Birch Reduction of but-2-yne
The above reaction shows the third step of birch reduction of but-2-yne in which but-2-yne radical reacts with sodium metal and forms a radical anion and then the radical anion reacts with ammonia to form butene.
Interesting Facts
Birch reduction may be a very important and useful reaction. It's used in the reduction of aromatic and non-aromatic compounds.
It's useful, particularly in the reduction of aromatic arenes due to its selectivity of reduction of certain double bonds.
Birch reduction is generally carried out at low temperatures.
Key Features of Birch Reduction
Birch reduction mainly involves the reduction of aromatic arenes.
Birch reduction occurs by the reaction of an aromatic arene with metal dissolved in ammonia and in the presence of alcohol.
Birch reduction involves radical anion and radical formation in its mechanism.
Birch reduction is stereospecific in reaction.
FAQs on Birch Reduction Mechanism Explained with Examples
1. What is the Birch reduction reaction?
Birch reduction is a chemical reaction used in organic chemistry to convert aromatic compounds into 1,4-cyclohexadienes. It uses an alkali metal like sodium or lithium dissolved in liquid ammonia, along with an alcohol, to partially reduce the aromatic ring in a very specific way.
2. What are the essential reagents for a Birch reduction?
The three main components needed for a successful Birch reduction are:
- An alkali metal, usually sodium (Na) or lithium (Li), which provides the electrons for the reduction.
- Liquid ammonia (NH₃), which acts as the solvent to dissolve the metal and create solvated electrons.
- An alcohol, like ethanol or t-butanol, which serves as a source of protons to complete the reaction.
3. What is the typical product when an alkyne undergoes Birch reduction?
When an alkyne is treated with the reagents for Birch reduction, it is reduced to a trans-alkene. This is different from other reduction methods, like using Lindlar's catalyst, which produce a cis-alkene. The formation of the trans product is a key feature of this reaction for alkynes.
4. How do substituents on an aromatic ring affect the outcome of a Birch reduction?
The type of group attached to the ring changes the reaction's result. If the group is electron-donating (like -CH₃), the carbons ortho and meta to it get reduced. If the group is electron-withdrawing (like -COOH), the carbon it's attached to (ipso) and the one opposite it (para) get reduced.
5. Why is liquid ammonia the required solvent for this reaction?
Liquid ammonia is crucial because it can dissolve alkali metals without reacting with them. This creates a special solution containing solvated electrons, which are the powerful reducing agents that attack the aromatic ring. No other common solvent can do this effectively, making liquid ammonia unique for this purpose.
6. What is the specific role of alcohol in the Birch reduction mechanism?
The alcohol acts as a proton source. During the mechanism, negatively charged intermediates are formed. The alcohol provides the necessary protons (H⁺) to neutralise these intermediates at the right moments, allowing the reaction to move forward to the final product. It is a much better proton donor than liquid ammonia alone.
7. Why does Birch reduction form a 1,4-diene instead of a more stable conjugated 1,2-diene?
This is controlled by the stability of the intermediates. The radical anion that forms during the reaction is most stable when the negative charge and the radical electron are as far apart as possible. This leads to protonation at the 'para' (or 4th) position, which locks the structure into a pathway that results in the non-conjugated 1,4-diene. The pathway to the conjugated product is less stable and therefore not formed.























