
Which of the following groups is sharp ortho and para directive?
A) $ - {{\text{C}}_6}{{\text{H}}_5}$
B) $ - {\text{OH}}$
C) $ - {\text{C}}{{\text{H}}_3}$
D) $ - {\text{Cl}}$
Answer
233.1k+ views
Hint: Here, all the groups are ortho and para directing as they are all electron donating groups .The $ - {\text{OH}}$ is the sharp ortho and para directing group as the lone pair present on oxygen group increase electron density at ortho and para positions.
Step-by-Step Explanation:
When mono-substituted benzene reacts with an electrophile, the electrophile can get attached to ortho, para or meta position on benzene. The electrophile if gets attached to the ortho or para position, it is called the ortho and para directing group. If it gets attached to the meta position it is called a meta directing group. The ortho and para directing groups are electron donating groups. Here, given all options are ortho and para directing. But $ - {\text{OH}}$ group is sharp ortho and para directing as the lone pair electron present in the Oxygen atom of the group increases electron density at ortho and para position so that the electrophile can easily get attached at these positions. The resonance structure of phenol is as follows-
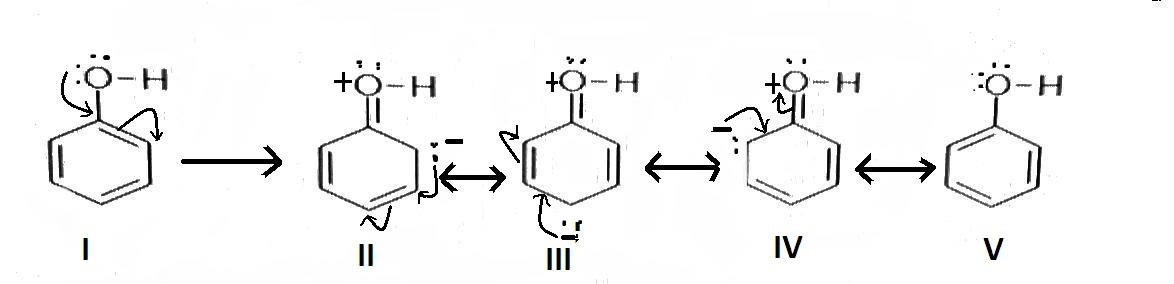
Hence correct answer is ‘B’.
Note: While ortho and para directing groups are electron donating groups, the meta directing groups are electron withdrawing groups. Halides are the exception of this rule because even when they are electron withdrawing groups they are still ortho and para directing.
Step-by-Step Explanation:
When mono-substituted benzene reacts with an electrophile, the electrophile can get attached to ortho, para or meta position on benzene. The electrophile if gets attached to the ortho or para position, it is called the ortho and para directing group. If it gets attached to the meta position it is called a meta directing group. The ortho and para directing groups are electron donating groups. Here, given all options are ortho and para directing. But $ - {\text{OH}}$ group is sharp ortho and para directing as the lone pair electron present in the Oxygen atom of the group increases electron density at ortho and para position so that the electrophile can easily get attached at these positions. The resonance structure of phenol is as follows-

Hence correct answer is ‘B’.
Note: While ortho and para directing groups are electron donating groups, the meta directing groups are electron withdrawing groups. Halides are the exception of this rule because even when they are electron withdrawing groups they are still ortho and para directing.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




