
Which of the following carboxylic acids undergoes decarboxylation most easily on heating?
A.
B. 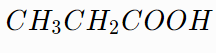
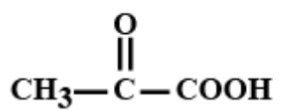
C.
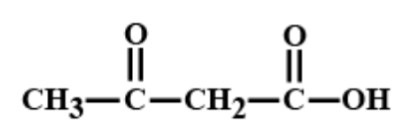
D.
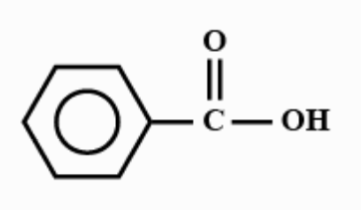
Answer
232.8k+ views
Hint : We know that a carboxylic acid has formula \[RCOOH\] where $R$ can be hydrogen benzene ring and hydrocarbon. Decarboxylation is a chemical reaction where removal of a carboxyl group takes place and releases carbon dioxide . Mostly decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. This reaction can be performed by heating carboxylic acid.
Complete step by step solution:
> Decarboxylation of any carboxylic acid depends upon the stability of carbanion. Carboxylic acids with beta keto systems are the most suitable carboxylic acid for the decarboxylation reaction. This is because the beta keto system stabilises the carbanion formation. $H - $ bonding makes decarboxylation reaction easy as it transfers acidic hydrogen into ketone oxygen, and eliminates carbon dioxide. Hence simple carboxylic acids which do not possess beta keto systems are harder to decarboxylate.
> We can identify $\beta - $ keto acid because these acids have ketone group at the second carbon from carbon from carboxylic acid. Here after observing all the options we got to know that the only option C i.e. $C{H_3}COC{H_2}COOH$ is $\beta - $ keto acid so it will go in decarboxylation easily on heating. The decarboxylation reaction of $C{H_3}COC{H_2}COOH$ on heating is given below;
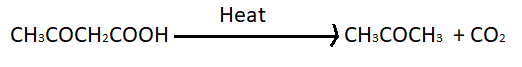
Note : We have approached this reaction by finding the stability of carboxylic acid . We also know that carboxylic acid undergoes decarboxylation most easily on heating if it is $\beta - $ keto acid because it becomes less stable than simple carboxylic acid. Hence we have found after observing the options that the $\beta - $ keto acid is option C. We can find $\beta - $ keto acid as it has a keto group next second carbon from carboxylic acid.
Complete step by step solution:
> Decarboxylation of any carboxylic acid depends upon the stability of carbanion. Carboxylic acids with beta keto systems are the most suitable carboxylic acid for the decarboxylation reaction. This is because the beta keto system stabilises the carbanion formation. $H - $ bonding makes decarboxylation reaction easy as it transfers acidic hydrogen into ketone oxygen, and eliminates carbon dioxide. Hence simple carboxylic acids which do not possess beta keto systems are harder to decarboxylate.
> We can identify $\beta - $ keto acid because these acids have ketone group at the second carbon from carbon from carboxylic acid. Here after observing all the options we got to know that the only option C i.e. $C{H_3}COC{H_2}COOH$ is $\beta - $ keto acid so it will go in decarboxylation easily on heating. The decarboxylation reaction of $C{H_3}COC{H_2}COOH$ on heating is given below;
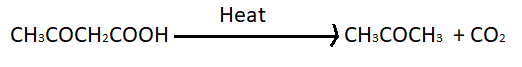
Note : We have approached this reaction by finding the stability of carboxylic acid . We also know that carboxylic acid undergoes decarboxylation most easily on heating if it is $\beta - $ keto acid because it becomes less stable than simple carboxylic acid. Hence we have found after observing the options that the $\beta - $ keto acid is option C. We can find $\beta - $ keto acid as it has a keto group next second carbon from carboxylic acid.
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Types of Solutions in Chemistry: Explained Simply

Difference Between Crystalline and Amorphous Solid: Table & Examples

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE General Topics in Chemistry Important Concepts and Tips

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




