
_______ is added to LPG to detect leakage.
(A) Propene
(B) Hexanol
(C) Isobutene
(D) Mercaptan
Answer
232.8k+ views
Hint: It is an organic gas of carbon, sulphur and hydrogen. It has a stink of rotten cabbages or smelly socks. It is found in the human body as a waste product of metabolism. It is also known as methanethiol. The chemical formula of the thiol group is \[-SH\].
Complete step by step answer:
- Mercaptan, also known as methanethiol (\[C{{H}_{3}}-SH\]) is added to LPG to detect leakage. It is a flammable and colourless gas.
- It emits toxic fumes and flammable vapours on heating.
- LPG is mainly composed of butane and propane and those are odourless.
- As we know that LPG is dangerous, if it leaks, it will cause fire and explosion.
- In that case, the natural gas mercaptan with the rotten smell of sulphur is added to the gas. - The smell helps to detect mere leakage of LPG which prevents any accident.
- It is an ideal gas to be added to odourless and flammable gases.
Structure of mercaptan:
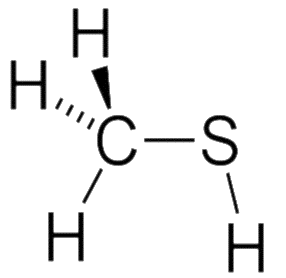
- It is a weak acid and tetrahedral in shape with carbon.
- It is used extensively in industries because it can be detected by the crowd even in small quantities.
So, the correct option is D.
Note: Mercaptan causes irritation when comes in contact with moist tissues like skin, eyes and nose. It creates problems like dizziness, nausea, vomiting, coma and even death. So it is toxic and should not be inhaled for a long time. It is also called “stenching gas” for its smell.
Complete step by step answer:
- Mercaptan, also known as methanethiol (\[C{{H}_{3}}-SH\]) is added to LPG to detect leakage. It is a flammable and colourless gas.
- It emits toxic fumes and flammable vapours on heating.
- LPG is mainly composed of butane and propane and those are odourless.
- As we know that LPG is dangerous, if it leaks, it will cause fire and explosion.
- In that case, the natural gas mercaptan with the rotten smell of sulphur is added to the gas. - The smell helps to detect mere leakage of LPG which prevents any accident.
- It is an ideal gas to be added to odourless and flammable gases.
Structure of mercaptan:

- It is a weak acid and tetrahedral in shape with carbon.
- It is used extensively in industries because it can be detected by the crowd even in small quantities.
So, the correct option is D.
Note: Mercaptan causes irritation when comes in contact with moist tissues like skin, eyes and nose. It creates problems like dizziness, nausea, vomiting, coma and even death. So it is toxic and should not be inhaled for a long time. It is also called “stenching gas” for its smell.
Recently Updated Pages
Area of an Octagon Formula Explained Simply

Absolute Pressure Formula Explained: Key Equation & Examples

Central Angle of a Circle Formula Explained Quickly

Difference Between Vapor and Gas: JEE Main 2026

Difference Between Atom and Molecule: JEE Main 2026

Carbon Dioxide Formula - Definition, Uses and FAQs

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Jan 21 Shift 1 Question Papers with Solutions & Answer Keys – Detailed Day 1 Analysis

JEE Main Response Sheet 2026 Released – Key Dates and Official Updates by NTA

JEE Main 2026 Answer Key OUT – Download Session 1 PDF, Response Sheet & Challenge Link

JEE Main Marks vs Percentile 2026: Calculate Percentile and Rank Using Marks

JEE Main 2026 Jan 22 Shift 1 Today Paper Live Analysis With Detailed Solutions

Other Pages
Happy New Year Wishes 2026 – 100+ Messages, Quotes, Shayari, Images & Status in All Languages

One Day International Cricket

Valentine Week 2026: Complete List of Valentine Week Days & Meaning of Each Day

List of Highest T20 Scores in International Cricket

Makar Sankranti Wishes: Happy Makar Sankranti Wishes in Marathi, Hindi, Kannada, and English

What is the Full Form of UGC? Detailed Guide for Students




