




Reduction Reaction - A Brief Description
The reduction process is simply the inverse of the oxidation process. Two hydrogens should be added during reduction if two hydrogens are removed during oxidation. Carbonyl compounds act as the oxidising agent, while hydrogen gas acts as the reducing agent in the presence of a metal catalyst. Other hydrogen-releasing reducing agents may also be used in this reaction. For example, sodium borohydride or lithium aluminium hydride can be used as reducing agents. LiAlH4 is one of the most powerful reducing agents, capable of efficiently reducing any carbonyl and some other functional groups.
Since the Al-H bond in LiAlH4 is polar, it can act both as a base and a nucleophile. When the hydride ion reacts, it has a nearly ionic character, leaving the hydrogen as a hydride ion, which is a carbonyl group, which is also a polar covalent bond, and the presence of the bond allows the H- addition to occur. Due to the presence of the polarised carbonyl group, aldehydes and ketones are highly reactive compounds. As a result, aldehydes and ketones exhibit a number of common reactions. This article focuses primarily on the reduction reaction of aldehydes and ketones.
Reduction of Aldehydes and Ketones to Alcohols
Aldehydes and ketones can be reduced to a number of compounds under different conditions by different reagents used for the reduction of aldehydes and ketones. Either of the specific reducing agents is required for the formation of alcohol from aldehydes or ketones. Sodium borohydride (NaBH4) or lithium aluminium hydride is the reducing reagent (LiAlH4). Even though the reagents sound complicated, the compounds' structures are quite simple.
Both reagents (use lithium tetrahydridoaluminate or sodium tetrahydridoborate) produce the same product. The end product is the same whether lithium tetrahydridoaluminate or sodium tetrahydridoborate is used. As a result, aldehyde reduction produces primary alcohol. Similarly, ketones produced during the process result in the formation of a secondary alcohol. This method is used for the preparation of the alcohol by reduction of aldehydes and ketones. Furthermore, regardless of the reagent, the end product remains unchanged.
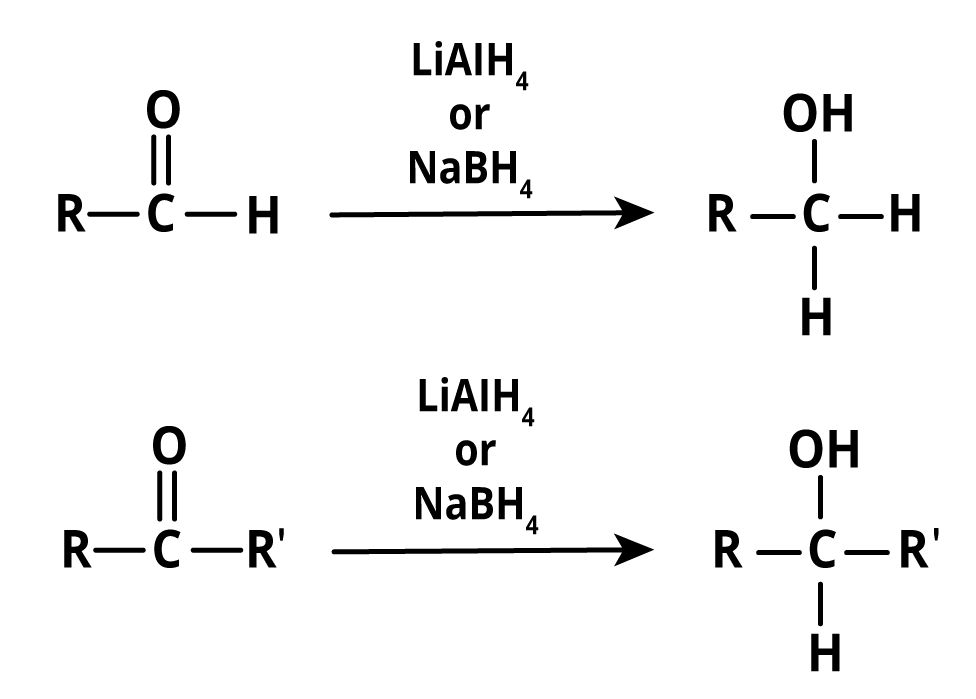
Image: Preparation of Alcohol by Reduction of Aldehydes and Ketones
Reduction of Aldehydes and Ketones into Hydrocarbon
To form a hydrocarbon, the carbonyl group in aldehydes and ketones can be reduced to methylene. The following methods are commonly used.
Clemmensen Reduction
In this method, aldehydes and ketones are heated with finely divided, amalgamated zinc in a hydroxylic solvent containing a mineral acid such as HCl. The amalgamated mercury-zinc serves only to provide a clean active metal surface and does not participate in the reaction. Substituents such as hydroxyl, alkoxy, and halogens are reduced first, followed by the reduction of the resulting unsubstituted aldehyde or ketone into the parent hydrocarbon.
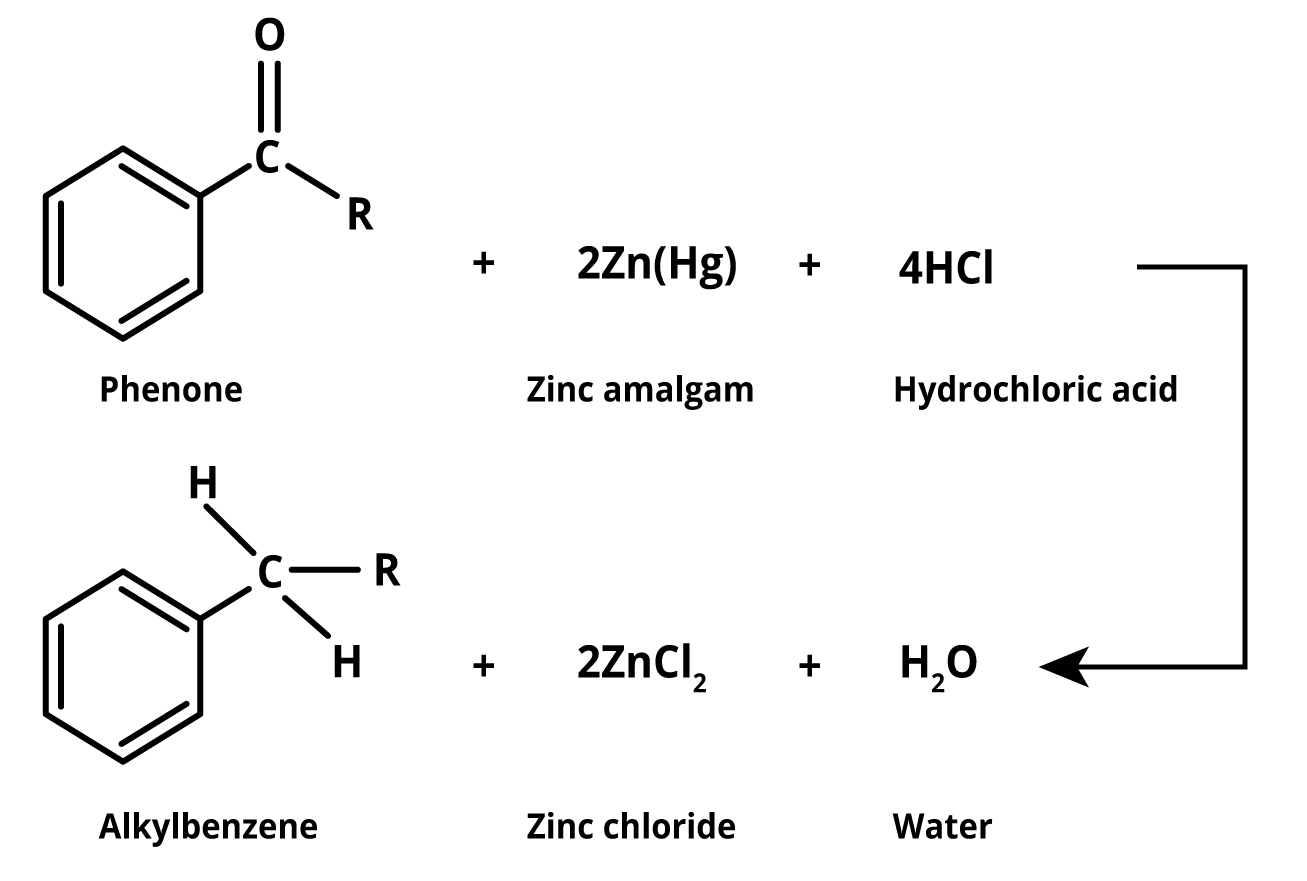
Image: Clemmensen Reduction
This reaction is commonly used to convert a carbonyl group to a methylene group. The reduction occurs at the zinc catalyst's surface. Alcohols are not postulated as intermediates in this reaction because subjecting the corresponding alcohols to the same reaction conditions does not result in alkanes. The Clemmensen Reduction mechanism is rationalised using the intermediacy of zinc carbenoids. It has a significant role in the synthesis of polycyclic aromatics and aromatics with unbranched side hydrocarbon chains. This reaction helps to reduce mixed aliphatic-aromatic carbonyl compounds into alkanes.
Wolff-Kishner Reduction
N. Kishner and L. Wolff independently discovered the Wolff-Kishner reduction in 1911. The Wolff-Kishner reduction is an organic Chemistry reaction that converts carbonyl functionalities into methylene groups. This reaction has a wide range of applications in organic synthesis, particularly for multi-walled carbon nanotubes. When an aldehyde or ketone reacts with excess hydrazine, a hydrazone derivative is formed, which when heated with base yields the corresponding hydrocarbon.
To achieve the required temperatures, a high-boiling hydroxylic solvent, such as diethylene glycol, is commonly used. The Tautomerisation of the initially formed hydrazone to an azo isomer is the mechanism of this useful transformation. Due to the highly basic conditions used in this reaction, it cannot be applied to base-sensitive compounds.
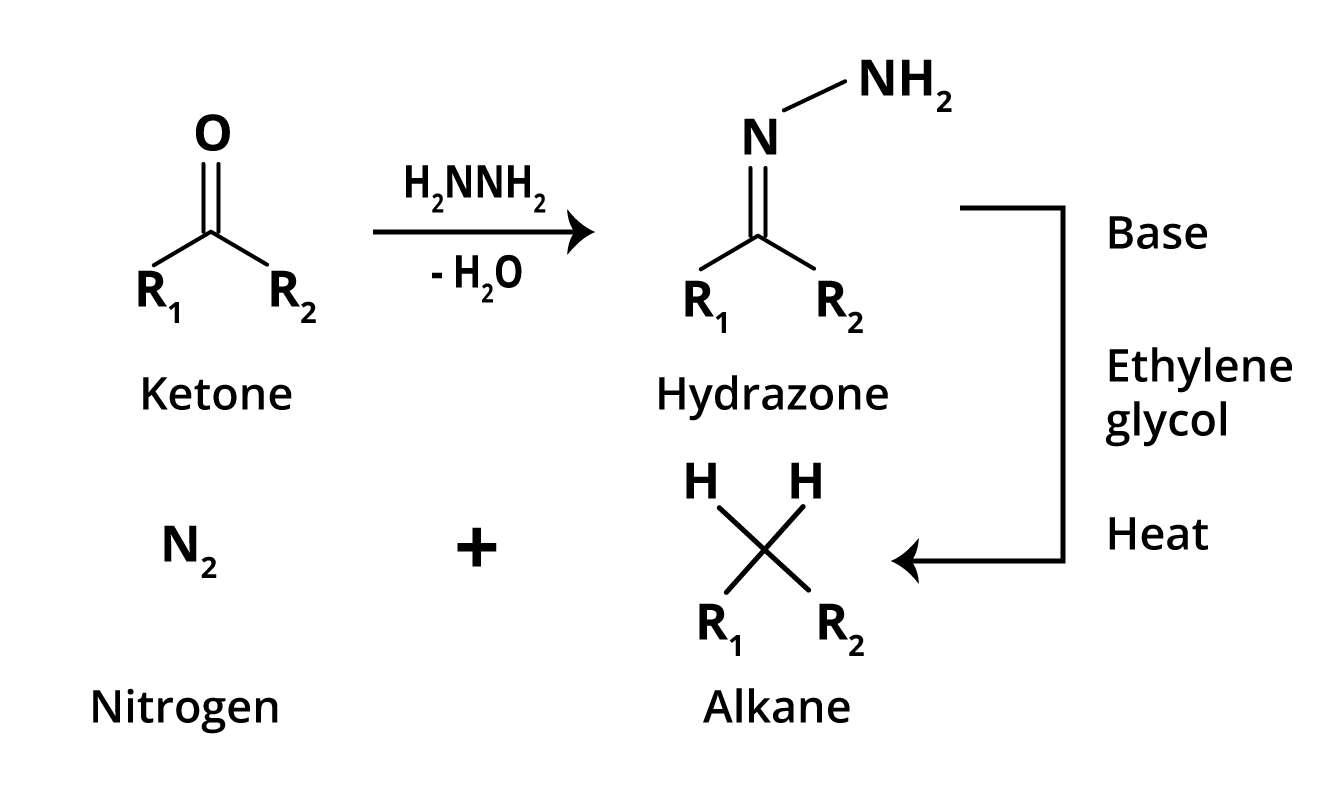
Image: Reduction of Wolff-Kishner
Conclusion
Due to the presence of the carbonyl group in aldehydes and ketone, they undergo various types of reactions. In this article, we study the reduction reaction of aldehydes and ketones. The most common and widely used reduction reaction of aldehyde and ketone is with Sodium borohydride (NaBH4) or lithium aluminium hydride.
In Clemmensen Reduction, when zinc amalgam is treated with conc. HCl, the carbonyl group of aldehydes and ketones is reduced in the -CH2 group. In Wolff-Kishner Reduction, when aldehydes and ketones are treated with hydrazine, the carbonyl group of the aldehydes and ketones is converted into a -CH2 group.
FAQs on Reduction of Aldehydes and Ketones for NEET
1. What is MPV reduction of aldehydes and ketones?
In Organic Chemistry, Meerwein-Ponndorf-Verley (MPV) reduces the conversion of ketones and aldehydes to their corresponding alcohols via aluminium alkoxide catalysis in the presence of sacrificial alcohol. The beauty of MPV reduction lies in its high chemoselectivity and use of a low-cost, environmentally friendly metal catalyst.
Eberwein and Schmidt discovered the MPV reduction in 1925, as did Verley separately. They discovered that a solution of aluminium ethoxide and ethanol could be used to convert aldehydes to their alcohols. Ponndorf modified the reaction to work with ketones and upgraded the catalyst to aluminium isopropoxide in isopropanol.
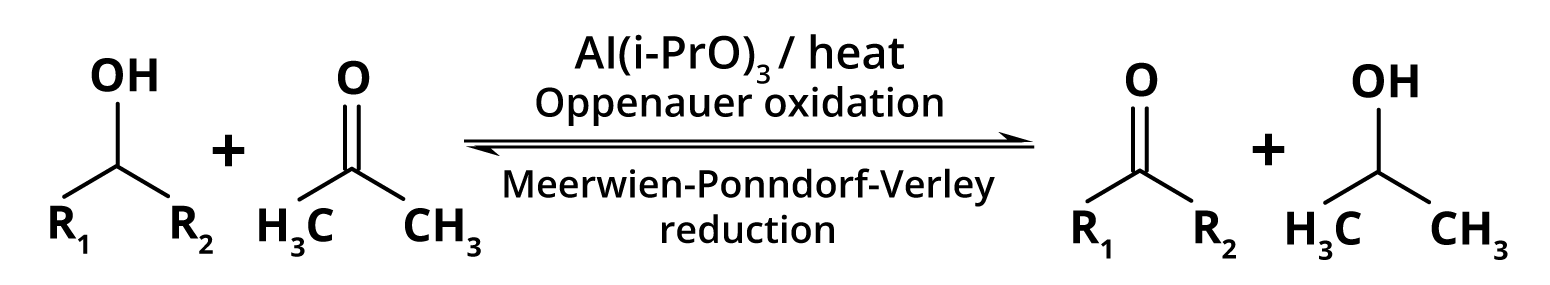
Image: Meerwein-Ponndorf-Verley (MPV) Reduction
2. How to distinguish between aldehydes and ketone with the Schiff test?
Ketones and aldehydes are carbonyl-containing organic compounds. These carbonic chemical compounds can be synthesised synthetically. The chemical structure of aldehydes and ketones is what distinguishes them. Schiff's test, Tollen's test, Fehling's test, Sodium hydroxide test, and other tests can help to differentiate them. Schiff's reagent is used to detect aldehydes and ketones. Sulphur dioxide gas is converted into a colourless solution known as Schiff's reagent solution by passing it through a magenta-coloured solution of p-rosaniline hydrochloride. As a result, Schiff's reagent is obtained for the detection. The aldehyde is then oxidised. As a result, the aldehyde group has a magenta colour.
























