




An Introduction to Periodic Table
The periodic table is an arrangement of all the elements known to man in accordance with their increasing atomic number and repeated chemical properties. Elements are placed in specific groups in the table. A standard form of the table contains 18 groups (vertical columns) and 7 periods (horizontal rows). All elements of the periodic table are represented in respective groups and periods. The periodic table is one of the most iconic and recognisable tools in the study of Chemistry. It has been around for over 150 years. The periodic table in general terms is the arrangement of chemical elements. So, these elements with similar properties can be grouped together.
Some Key Characteristics of Groups in the Periodic Table
There are nine groups in the modern periodic table and they are represented by roman numerals such as I, II, III, IV, V, Vi, VII, VIII, and zero. The modern periodic table group names and how they got the names will be discussed later in the article.
Groups I to VII are further divided into two subgroups A and B, Group VIII consists of three sets, each one containing three elements.
Inert gases or noble gases are placed in zero group.
The valency of an element in a group is equal to the group number.
The elements of the groups which resemble the typical elements are called normal elements. For example, IA, IIA, IIIA, IVA, VA, VIA, and VIIA group elements are normal elements.
Those elements of the groups which do not resemble the typical elements are called transition elements. For example, IB, IIB, IIIB, IVB, VB, VIB, VIIB, and VIII group elements are transition elements. Hydrogen is placed in both IA and VIIA groups.
There are a total of 18 different groups in the periodic table. These are as follows:
Group 1: Alkali metals group (hydrogen not included),
Group 2: Alkaline earth metals group,
Group 3-12: Transition and Inner transition metals group,
Group 13: Boron group,
Group 14: Carbon group,
Group 15: Nitrogen group,
Group 16: Oxygen group,
Group 17: Halogen group,
Group 18: Noble gases group.
Few groups are named by taking the reference of the first element of the group. Example: Carbon family, Boron family, etc.
Atomic weight, atomic size, electropositive character, and metallic character of elements increase down the group.
Ionisation potential, electron affinity, and electronegativity of elements decrease down the group.
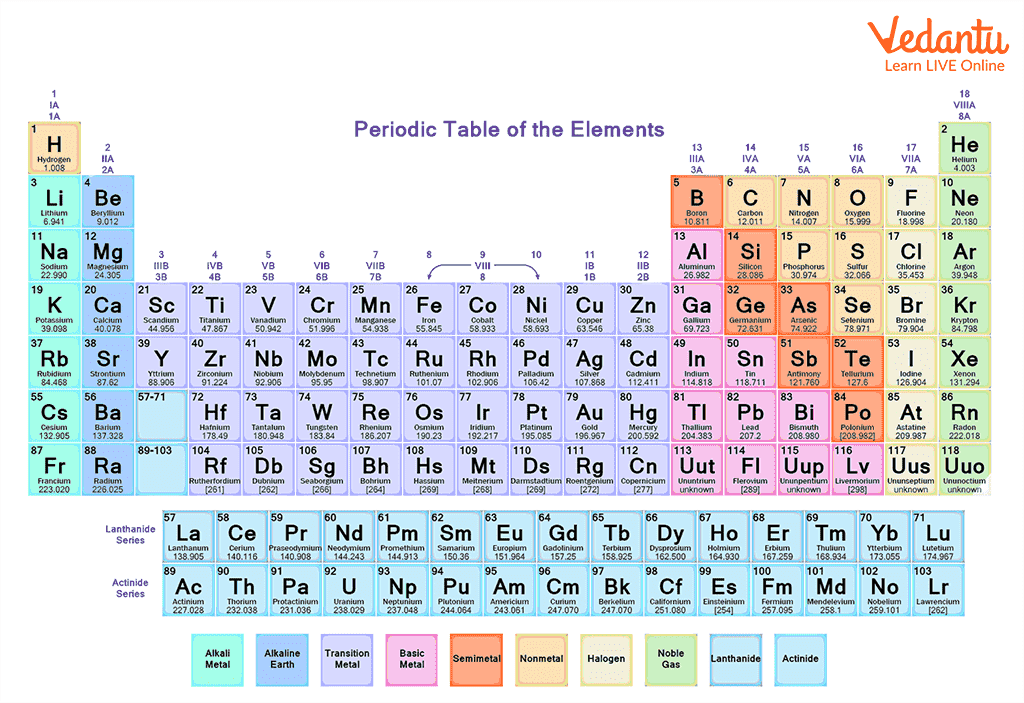
Periodic Table
Groups of the Periodic Table
Group 1:
Group 1 are the alkali metals. It is a group of 6 elements. This group spans from Lithium (Li) to Francium (Fr). They are named as alkali metals because when they react with water, they form compounds called alkalies (i.e., hydroxide compounds of these elements). For instance, sodium hydroxide and potassium hydroxide.
Few Characteristics of Group 1
Less dense than other metals.
One loosely bound valence electron.
Highly reactive, with reactivity increasing moving down the group.
The largest atomic radius of elements in their period.
Low ionisation energy.
Low electronegativity.
Group 2:
The second group of elements in the periodic table are known as the alkaline earth metals. These elements include beryllium, magnesium, calcium, strontium, barium, and radium. The alkaline earth metals are so named because they all have properties that make them similar to the alkali metals in the first group of the periodic table. Like the alkali metals, the alkaline earth metals are all highly reactive. They also share some other common properties, such as being soft and having low densities.
Few Characteristics of Group 2
Two electrons in the valence shell.
Readily form divalent cations.
Low electron affinity.
Low electronegativity.
3rd to 12th Group (Transition Elements):
The d-block elements, also called the transition elements, are located in the middle of the periodic table. It spans from Titanium (Ti) through Copernicium (Cn). The transition metal group consists of 38 elements in the Periodic Table.
Few Characteristics of Transition Elements
Hard but malleable. Have a lustre.
Much less reactive than alkaline-earth metals.
Good conductors of heat and electricity.
Possess a high density, high melting point and boiling points.
Possess a high density and high melting points and boiling points.
All transition metals occur in the solid state. Mercury is the only metal that exists in the liquid state.
13th Group (Boron Group):
The boron group is a column of elements in the periodic table that includes boron (B), aluminium (Al), gallium (Ga), indium (In), and thallium (Tl). The key property of the group is that every one of the elements has three electrons in the peripheral shell of their nuclear structure. Boron, the lightest of these elements, is non-metal, yet alternate individuals from the group are brilliant white metals.
Few Characteristics of the Boron Group
The elements of group 13 react with halogens to form trihalides. One atom of the element of the boron family reacts with 3 atoms of halogens.
Trihalides hydrolyse in water and show covalent bonding.
Trihalides are good Lewis acids due to the deficiency of electrons.
From boron to aluminium, the electronegativity first decreases but slightly increases as we go further down.
The metallic character of the boron family increases as we move down the group.
While moving down the group of the boron family, the oxidation state of +1 is much more stable than the oxidation state of +3. This property is because of the inert pair effect.
Due to the icosahedral structure of boron, it has an extremely high melting point and Gallium (Ga) has the least melting point.
14th Group (Carbon Group):
The carbon group is a periodic table group consisting of carbon, silicon, germanium, tin, lead, and flerovium. This group lies in the p-block of the periodic table. The members of this group have four valence electrons in their outermost shell. As all the elements in group 14 have 4 electrons in the outermost shell, the valency of group 14 elements is 4. They use these electrons in the bond formation in order to obtain octet configuration.
Few Characteristics of the Carbon Group
As you move down the periodic table in the carbon family, the atomic radius and ionic radius increase.
The radii of group 14 elements are smaller than that of group 13 elements.
As you move down the periodic table in the carbon family, electronegativity and ionisation energy decrease.
The general oxidation states exhibited by the group 14 elements are +4 and +2.
Group 14 (carbon family) elements have much higher melting points and boiling points than the group 13 elements.
Down the group, the metallic character increases. C is non-metals, Ge and Si are metalloids, and Sn and Pb are soft metals with low melting points.
15th Group (Nitrogen Group):
The nitrogen group is a category of elements in the periodic table. The Group 15 elements as it would move down a group, starting with the lightest element and finishing with the heavy ones. The first two elements in the group, nitrogen (N) and phosphorus (P) are nonmetals; the remaining three elements are arsenic (As), antimony (Sb), and bismuth (Bi).
Few Characteristics of the Nitrogen Group
As we move down the group, the radius of the atom increases, and therefore the Ionisation energy decreases due to the weaker hold of the nucleus.
The electronegativity value decreases down the group with increasing atomic size.
Firstly, nitrogen is a gas, but as you move down, there is a significant increase in the metallic character of the elements. Nitrogen and phosphorus are non-metals; arsenic and antimony are metalloids and bismuth is a metal.
The common oxidation states of these elements are -3, +3, and +5.
In general, the boiling points also show an increasing trend as you move down.
Sixteenth Group (Chalcogens):
The chalcogens are a group of elements in the periodic table consisting of oxygen (O), sulphur (S), selenium (Se), tellurium (Te), and polonium (Po). All these elements belong to the 16th group of the periodic table. All chalcogens have a total of 6 electrons in their respective valence shells. These elements are also known as ore-forming elements since a large number of metals are known to exist in the form of sulphides or oxides in the Earth’s crust.
Few Characteristics of the Chalcogens
They are all volatile, meaning they easily form gases at room temperature.
The regular oxidation states shown by the chalcogens include -2, +2, +4, and +6.
The atomic radii or the ionic radii of elements increase while progressing down a group. The chalcogen with the lowest atomic radius and ionic radius is oxygen, whereas the chalcogen with the largest atomic/ionic radius (excluding livermorium) is polonium.
The melting and boiling points of the elements also increase while progressing down a group. Among the chalcogens, oxygen is known to have the lowest melting and boiling point.
Seventeenth Group (Halogens):
The halogens are a group of five elements in the periodic table: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They are all non-metals and have similar properties.
Few Characteristics of the Halogens
All of the halogens are highly reactive and form compounds with other elements.
Fluorine is the most reactive of all the elements and is used in industry to make Teflon.
The group of halogens is the only periodic table group that contains elements in all three familiar states of matter at standard temperature and pressure.
Halogens have seven valence electrons because halogens have one electron missing; they form negative ions and are highly reactive.
All of the halogens are poisonous and can be dangerous if inhaled or ingested.
Fluoride is added to drinking water in some areas to help prevent tooth decay, but too much fluoride can cause health problems.
Eighteenth Group (Noble Gases):
The noble gases are a group of elements in the periodic table. The noble gases include helium, neon, argon, krypton, xenon, and radon. They are all located in the right-most column of the table, and they all have very low reactivity. This is because their outermost electron shells are full, so they don't need to react to achieve stability.
Few Characteristics of the Noble Gases
The members of Group 18 have very small atomic radii. Atomic radii of noble gases increase down the group with an increase in atomic number due to the addition of new shells.
They are all very unreactive and have low melting and boiling points. The noble gases are all colourless, odourless, and tasteless.
The melting and boiling points increase on moving down the group with an expansion in the extent of the Van der Waals forces of attraction.
Helium has the lowest boiling point among the elements in group eighteen.
Ionisation enthalpies decrease step by step on moving down the group with an expansion in the nuclear size.
In this article, we have discussed groups of the periodic table. A periodic table is a tabular arrangement of the chemical elements, organised based on their atomic numbers, electron configurations, and recurring chemical properties. Elements in the same column (group) have similar valence electrons and tend to exhibit similar chemistry. Here, we also discussed the few characteristics of the groups and discussed in detail about the groups. Hope the article was helpful!
Essential Study Materials for NEET UG Success
FAQs on Periodic Table- NEET Important Topic
1. How were elements classified in the modern periodic table?
The elements of the modern periodic table are as follows:
There are alkali and alkaline earth metals which are present on the left side of the periodic table and have elements that are highly reactive. The elements of the 1st group have one electron in the valence shell and the elements of the 2nd group have two electrons.
There are transition metals that occupy the periodic table’s centre and show the properties of the metals. They start from 3-12. Some of them are also placed separately in the bottom two rows and are named as lanthanides and actinides according to their properties.
There are metalloids and nonmetals. Metalloids are present diagonally in the right side of the table and differ from the non-metals present in the right side. They express both the properties of nonmetals and metals and are so named.
Lastly, there are noble gases which are present at the extreme right of the table. They are the 18th group and have filled valence shells and have properties like non-reactivity.
2. State the importance of the periodic table.
The periodic table is the basis of all chemistry fundamentals. Since Chemistry covers our environment, the fundamentals of everything are now dependent on it. Consider anything concrete that does not involve Chemistry. That is impossible! Starting with the air you breathe and ending with the ashes you become after cremation, everything concrete has a chemical makeup and, as a result, Chemistry is involved. The various elements of the periodic table are present around us in the form of solid, liquid or gases. Some of these elements provide us essential nutrients for survival while some are utilised in other scientific activities.
3. Is the periodic table important for the NEET exam?
Yes, the periodic table is important for NEET. It organises elements in a way that helps you understand their relationships, predict their properties, and balance chemical reactions easily.
4. Which element is the oldest?
Hydrogen and helium are the first and most common elements in the universe.
5. What is the latest element discovered?
The newest element is Oganesson (Og). It was added to the periodic table in 2016 and is the 118th element. It is very rare and breaks down in less than a millisecond.
6. What is the rarest element on Earth?
Astatine is the rarest element. It is radioactive, and its longest-lasting form exists for only about 8.1 hours. Scientists estimate that there is less than one gram of astatine in the entire Earth's crust.
7. What is the heaviest element ever made?
The heaviest element created so far is oganesson (element 118). Scientists first made it in 2002. Hiromitsu Haba, a researcher in Japan, calls this discovery "truly groundbreaking."
























