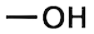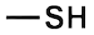An Introduction to the Functional Group
In the field of organic chemistry, functional groups are the substituent atoms or groups of atoms that are attached to specific molecules. These moieties i.e, the part of the molecule which can be found in many other molecules as well, are responsible for the chemical reactions that the molecule they are attached to participate in.
A group of atoms or bonds inside a substance that is responsible for the substance’s unique chemical reactions in organic chemistry is known as a functional group. Despite the chemical in which it is found, the same functional group will behave similarly and experience comparable reactions.
What are the Functional Groups?
A functional group is a group of atoms in a molecule that is responsible for the chemical properties of that molecule. They are the parts of the molecule that interact with other molecules and determine how the molecule will react with other substances. Functional groups can be found in both organic and inorganic molecules, and they play an important role in chemistry.
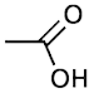
There are many different types of functional groups, each with its own unique properties. Some common examples include alcohols, amines, carboxylic acids, and ketones. The specific properties of a functional group depend on its structure, which is determined by the types of atoms that make up the group. For example, alcohols contain a hydroxyl group (OH), which gives them their characteristic bitter taste. Carboxylic acids contain a carboxyl group (COOH), which makes them acidic.
Importance of Functional Groups
Functional groups are important because they give molecules their chemical identity. They determine how a molecule will interact with other molecules. They can be used to predict the behaviour of a substance in certain reactions. Additionally, functional groups can be used to classify chemicals into different categories. For example, all alcohols have the same general structure (a carbon atom bonded to two hydrogen atoms), but they can be further classified based on the type of alcohol (e.g., methanol or ethanol). This classification system helps chemists understand the similarities and differences between different chemicals.
Common Functional Groups
Hydrocarbons
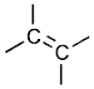
Alkanes, alkenes, and alkynes are referred to as hydrocarbyl groups since they contain only carbon and hydrogen atoms. Alkanes, alkenes, and alkynes are represented by the symbol R. However, they may vary in the types of bonds between two carbon atoms, such as double or triple bonds.
The reactivity of these groups changes due to the nature of the carbon-carbon bond. Some groups are made up of a long, branched alkane or a ring-structured alkane, which are assigned specific names. Few examples include names such as cyclohexyl. Hydrocarbon functional groups may have an ionic charge on them. The positively charged structures are referred to as carbocations, whereas the negatively charged hydrocarbons are called carbanions.
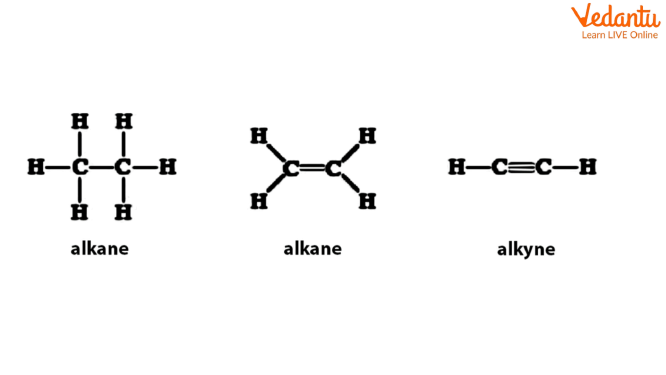
Hydrocarbons
Haloalkanes
Haloalkanes, also known as alkyl halides, are functional groups that contain a carbon-halogen bond. The prefix 'halo-' is used to denote a halogen. For example, the compound CH3F can be referred to as fluoromethane, with the prefix fluoro. The halide suffix is used to denote a halogen. For example, fluoromethane (CH3F) can also be referred to as methyl fluoride, with the suffix fluoride.
The strength and stability of the carbon-halogen bond vary depending on the halogen. The carbon-iodine bond in alkyl iodides, for example, is quite weak, whereas the carbon-fluorine bond in alkyl fluorides is quite strong and stable. With the exception of these alkyl fluorides, all alkyl halides readily undergo elimination or nucleophilic substitution reactions.
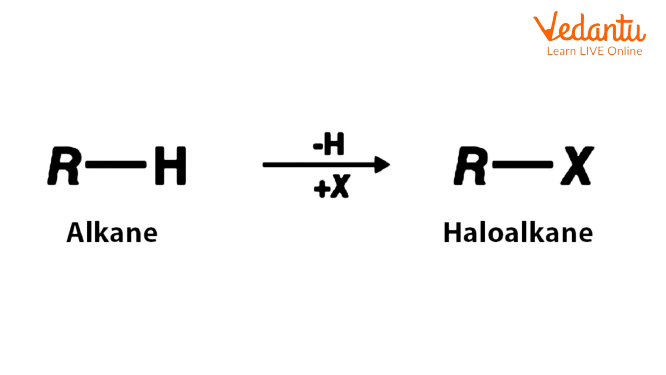
Haloalkanes
Oxygen-containing Functional Groups
The properties of the functional groups containing a carbon-oxygen bond are entirely dependent on the hybridisation of the carbon-oxygen bond. This can be explained by the electron-donating effect of the sp3 hybridisation of oxygen which can be observed in alcohols in sharp contrast with the electron-withdrawing effect of the sp2 hybridised oxygen, which can be observed in the carbonyl groups which contain a carbon-oxygen double bond.
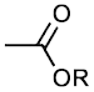
The suffixes used in the nomenclature of compounds which have a functional group containing a C-O bond are tabulated below, along with examples. It can be noted that only a few common functional groups containing the carbon-oxygen bond are tabulated above. Many uncommon groups with complex compositions such as acetal groups (RCH(OR’)(OR’’), or ketal groups RC(OR’)(OR”)R”.
Nitrogen-containing Functional Groups: The substituent groups that contain nitrogen may also contain carbon-oxygen bonds.
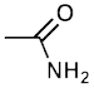
For example, the amide functional group has the formula R-(CO)-NR2 and therefore has a carbonyl carbon which is bonded to a nitrogen atom, which is in turn bonded to two other alkyl groups. Some common functional groups that contain nitrogen are tabulated below, along with the suffixes for their nomenclature. It can be noted that many nitrogen-containing functional groups with comparatively large sizes have not been mentioned in the tabular column given above, the pyridine derivatives with the formula RC5H4N, for example.
Alcohols: Alcohols are molecules that contain a hydroxyl group (-OH) attached to an alkyl group (a carbon chain). The simplest alcohol is methanol $\left( C{{H}_{3}}OH \right)$, which consists of a methyl group\[(-C{{H}_{3}})\] bonded to a hydroxyl group.
Phenols: Phenols are another type of oxygen-containing functional group. Phenols consist of a hydroxyl group bonded to an aromatic ring (a ring of carbon atoms with alternating double and single bonds).
Ethers: Ethers are a class of organic compounds that contain an oxygen atom bonded to two alkyl or aryl groups. Ethers typically have the general formula\[R-O-{{R}^{'}}\], where R and ${{R}^{'}}$ represent the alkyl or aryl groups. Ethers can be classified as either symmetrical or asymmetrical, depending on whether the two alkyl groups are identical.
The physical properties of ethers depend on both the size and nature of the alkyl groups attached to the oxygen atom. Generally speaking, ethers are less volatile than similar hydrocarbons (molecules with only carbon and hydrogen atoms) due to their higher boiling points. This property makes them useful as solvents for high-boiling point chemicals such as dyes and resins. Additionally, ethers tend to be less flammable than hydrocarbons due to their lower vapour pressure (the pressure at which a liquid boils).
The chemical properties of ethers are largely determined by the type of bond between the oxygen atom and the alkyl groups (termed an "ether linkage"). The most common type of ether linkage is called an "ether bridge," which results in a three-membered ring structure known as an oxetane. This type of linkage is found in many biologically important molecules such as cholesterol and steroids. Other types of ether linkages include those formed by ring opening (as in cyclic ethers) or by replacement of one H atom with another functional group (as in alkoxyamines).
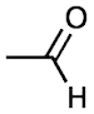
Aldehydes: Aldehydes are a class of organic compounds containing a carbonyl group bonded to at least one hydrogen atom. The general structure of an aldehyde is\[R-CHO\], where R is an alkyl or substituted alkyl group. Aldehydes are important in many areas of chemistry, including organic synthesis, biochemistry, and materials science.
The carbonyl group in an aldehyde is planar and trigonal, with the carbon atom bonded to two oxygen atoms (one double bond and one single bond). The \[C-O-C\] bond angle in an aldehyde is 120°. The carbonyl group is polar because the electronegativity of oxygen is greater than that of carbon. This results in the oxygen atom having a partial negative charge and the carbon atom having a partial positive charge.
Aldehydes are named using the IUPAC nomenclature system by replacing the ending -e of the corresponding alkane with -al. For example, the simplest aldehyde, methanal (also known as formaldehyde), has the chemical formula \[C{{H}_{2}}O\]. The second-simplest aldehyde is ethanal (also known as acetaldehyde), with the chemical formula\[{{C}_{2}}{{H}_{4}}O\].
Aromatic Aldehydes: An aromatic aldehyde is an organic compound containing both an aromatic ring and a terminal carbonyl group. The general structure of an aromatic aldehyde is \[Ar-CHO\], where Ar represents an aromatic ring such as benzene or naphthalene.
Aromatic aldehydes are important building blocks in organic synthesis and have numerous applications in industry and commerce. The most well-known aromatic aldehyde is vanillin, which imparts flavour to vanilla beans and other food products
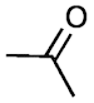
Ketones: Ketones are a class of organic compounds that contain the carbonyl group, C=O. The carbon atom of the carbonyl group is bonded to two other atoms, carbon. Ketones are named by identifying the alkyl groups attached to the carbonyl carbon atom and then adding the suffix "-one". For example, the simplest ketone is acetone,\[C{{H}_{3}}-CO-C{{H}_{3}}\].
Sulphides: The sulphides functional group is made up of one sulphur atom and two oxygen atoms. This group is found in a variety of organic molecules, including some amino acids and enzymes. The presence of the sulphides functional group gives molecules their characteristic smell, as well as making them more reactive than other functional groups. The reactivity of the sulphides group means that it can easily form bonds with other molecules, which can lead to the formation of new compounds.
Rule for Naming
The IUPAC nomenclature system is a set of rules that chemists use to name chemical compounds. The rules are designed to give each compound a unique and unambiguous name.
There are five main rules for naming organic compounds using the IUPAC system:
Find the longest continuous chain of carbon atoms in the compound. This is the "parent chain".
Identify the functional groups present in the compound and determine their highest priority.
Based on the parent chain and functional groups, determine the suffix of the compound (-ane, -ene, -yne, etc.).
Number the carbons in the parent chain so that the functional group with the lowest number has the lowest priority.
Name each substituent group by its appropriate prefix (methyl-, ethyl-, propyl-, etc.) and indicate its position on the parent chain with a number preceded by a dash (-).
Summary
In this article, we have learnt about functional groups. In the field of organic chemistry, functional groups are the substituent atoms or groups of atoms that are attached to specific molecules. A group of atoms or bonds inside a substance that is responsible for the substance’s unique chemical reactions in organic chemistry is known as a functional group. Despite the chemical in which it is found, the same functional group will behave similarly and experience comparable reactions. Here, we have also discussed the importance of functional groups along with some common examples.
FAQs on Nomenclature of Organic Carbon and Its Compound Functional Groups - NEET Important Topic
1. How do IUPAC names differ from common names?
The IUPAC nomenclature is the standardised name given to the organic compounds using official naming rules. Opposed to that, common names are older names given to the organic compounds, which are not official, but sometimes they are used. There is no explicit rule for writing the common names of the organic compound, and they are used interchangeably with IUPAC names, so one must get acquainted with both naming conventions terms.
2. How many common functional groups are there in organic chemistry?
Organic chemistry is a vital and huge part of chemistry. The main element of an organic compound is carbon atoms. Depending on the carbon bond structures and arrangements, the different carbon bondings and positionings are called functional groups. One or more functional groups form every organic compound. The functional groups have individual names based on their carbon atoms and bond structures. There are nine common functional groups in organic chemistry. All the common groups are named following the IUPAC nomenclature rules and priority order of functional groups in IUPAC nomenclature. The common functional groups of organic chemistry are aldehyde, amine, hydroxyl, ketone, phenyl, amino, ether, amide, ester.
3. Mention the basic principles of IUPAC naming.
IUPAC nomenclature is a general naming process for all organic compounds. IUPAC names of organic compounds are necessary to avoid long names of the compounds. Also, IUPAC names should be convenient for identification. Considering all these factors, the basic principles of IUPAC naming are: the parent hydrocarbon chain should have maximum branches, maximum substituents, maximum length, maximum single, and multiple bonds. The parent functional group should have the highest order of precedence. The side chains of organic compounds, which are not present in the parent chain, should be determined correctly. The numbering of chains and bonds should follow the priority order.