Stepwise Answers & Key Diagrams for Particulate Nature of Matter
FAQs on NCERT Solutions For Class 8 Science Chapter 7 Particulate Nature Of Matter - 2025-26
1. What is the particulate nature of matter according to Class 8 Science Chapter 7?
The particulate nature of matter explains that all matter is made up of tiny particles which are in constant motion. Key points include:
- Matter consists of particles too small to be seen with the naked eye.
- These particles can be atoms or molecules.
- Arrangement and movement of these particles determines the state (solid, liquid, gas).
- This concept forms the foundation for understanding NCERT Solutions for Class 8 Science Chapter 7.
2. How do NCERT Solutions for Class 8 Science Chapter 7 help in exam preparation?
NCERT Solutions provide stepwise answers, cover key definitions, diagrams, and exercise questions, making exam preparation systematic. Main benefits include:
- Structured, exam-oriented answers matching CBSE marking schemes.
- Covers intext, back exercise, and exemplar questions.
- Helps in practicing diagrams and definitions for full marks.
- Available in free PDF form for offline revision.
3. Are diagrams and definitions mandatory in answers for Class 8 Science Chapter 7?
Including diagrams and definitions is highly recommended for Class 8 Science Chapter 7. They help:
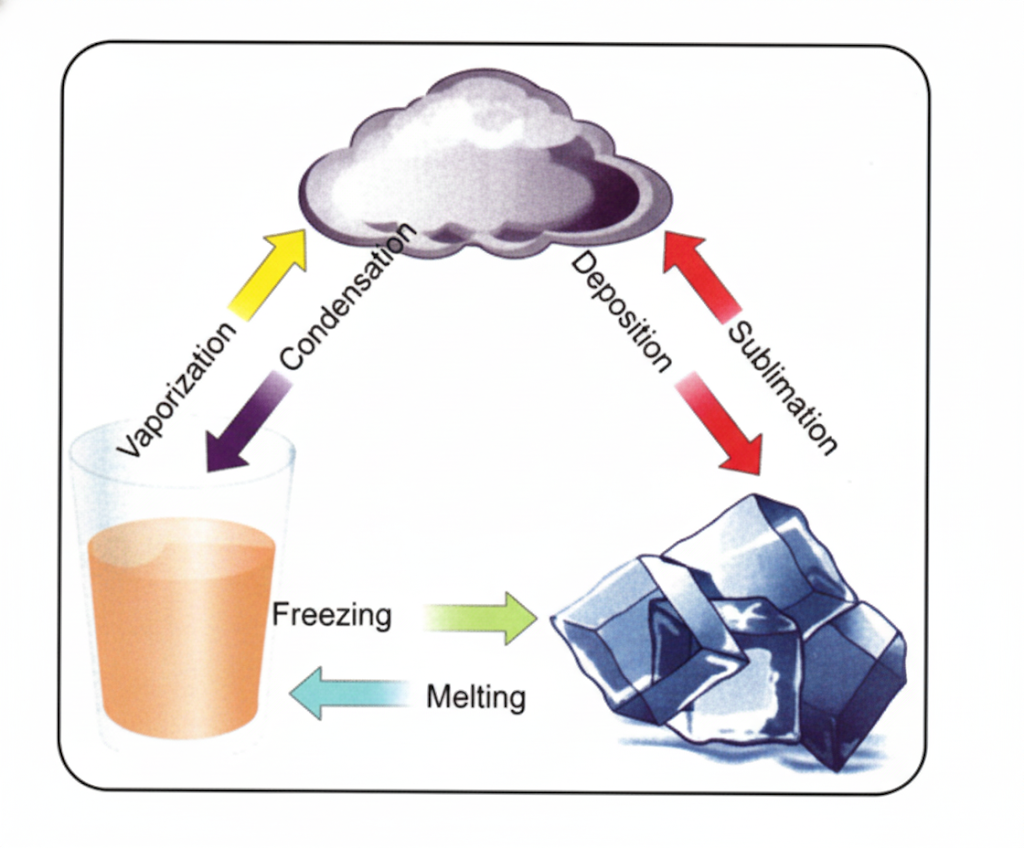
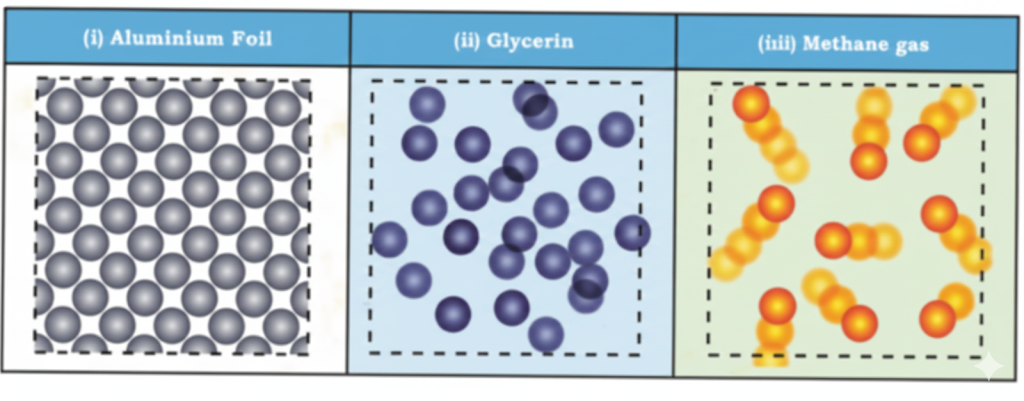
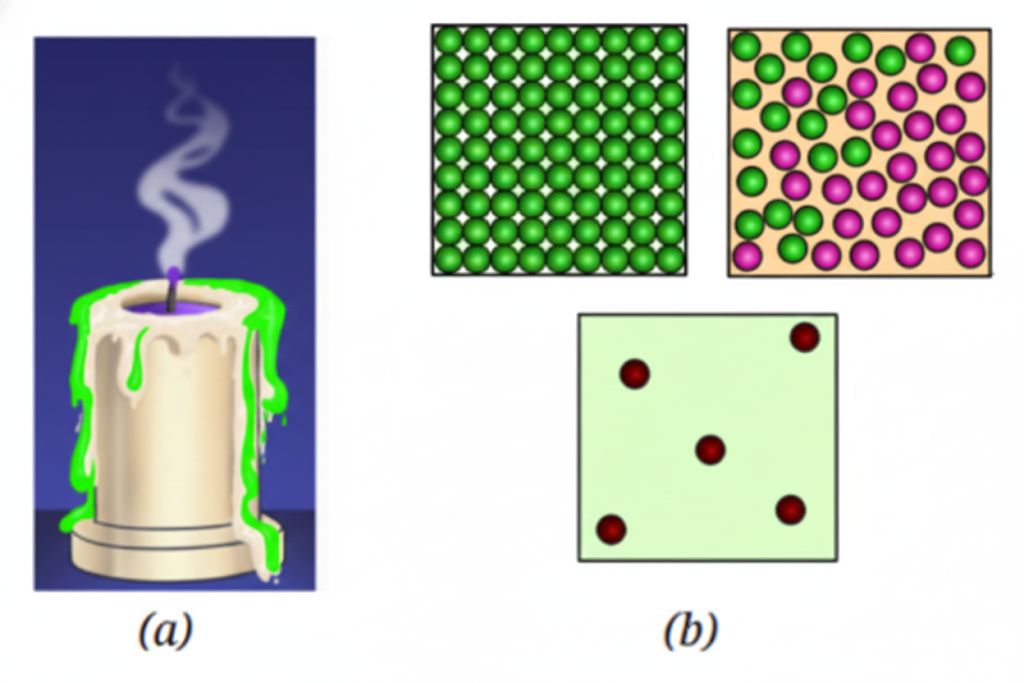
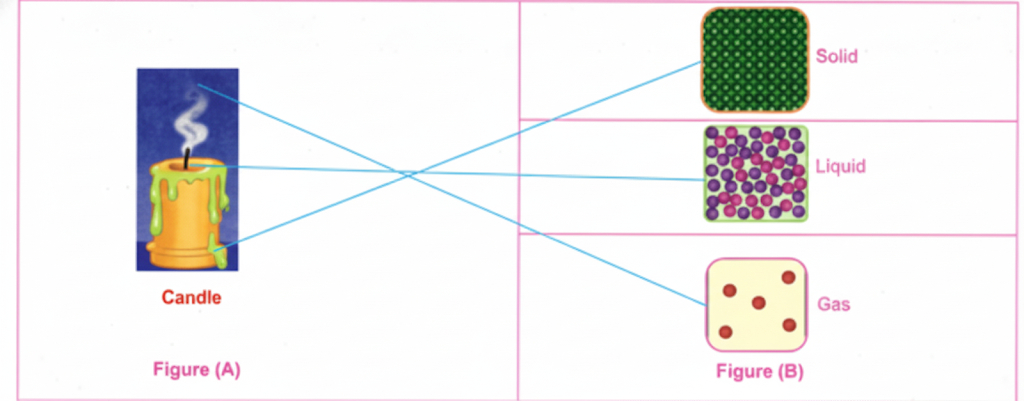
- Clarify concepts visually (e.g., arrangement of particles in solids, liquids, gases).
- Score easy marks in CBSE exams.
- Follow exam presentation tips as per the NCERT Solutions for Particulate Nature of Matter.
4. What are the key definitions students must remember in Chapter 7: Particulate Nature of Matter?
Some important definitions for Class 8 Science Chapter 7 are:
- Atom: The smallest unit of matter.
- Molecule: A group of two or more atoms bonded together.
- Diffusion: The process by which particles intermingle due to their motion.
- Solid, Liquid, Gas: States of matter distinguished by particle arrangement and movement.
5. Where can I download the NCERT Solutions PDF for Class 8 Science Chapter 7?
You can download the free PDF of NCERT Solutions for Class 8 Science Chapter 7: Particulate Nature of Matter on educational platforms or official study portals. Benefits include:
- Offline study at any time.
- Easier revision of structured answers and diagrams.
- Access to stepwise, syllabus-aligned solutions.
6. How should I structure long answers in Class 8 Science Chapter 7 for better marks?
To score well, structure long answers as follows:
- Start with a definition or introduction.
- List key points in sequence or steps.
- Include labelled diagrams if possible.
- Conclude with a summary or final statement.
- Use keywords like 'particulate nature', 'atoms', 'states of matter', and underline them in your answer sheet.
7. What are some common mistakes to avoid in writing answers for this chapter?
To maximize your score in Class 8 Science Chapter 7, avoid these mistakes:
- Missing key definitions, diagrams, or steps.
- Incorrectly labelling diagrams or omitting labels.
- Writing overly brief or incomplete answers.
- Skipping essential keywords or not underlining them in exams.
- Not following the NCERT stepwise solution approach.
8. What topics or questions are most likely to come from Chapter 7: Particulate Nature of Matter in exams?
Frequently asked exam topics from Class 8 Science Chapter 7 include:
- States of matter and their characteristics.
- Definitions (atom, element, molecule, diffusion).
- Differences between solids, liquids, and gases.
- Diagrams showing arrangement of particles in different states.
- Intext and back exercise questions from the NCERT textbook.
9. Are NCERT Solutions enough for Class 8 Science exams?
NCERT Solutions for Class 8 Science Chapter 7 are sufficient for most CBSE school exams as they:
- Cover all textbook exercises and intext questions.
- Follow the latest CBSE marking scheme.
- Help in understanding concepts, definitions, and the format required for full marks.
- For higher-order practice, refer to NCERT Exemplar Solutions and extra questions.
10. How can I quickly revise the main concepts of Chapter 7 before an exam?
To revise quickly for Class 8 Science Chapter 7:
- Read through flash notes listing definitions and key points.
- Practice labelling diagrams of particles in different states.
- Attempt NCERT back exercise and intext questions.
- Use a 1-day or 3-day revision planner as suggested in top study guides.
- Highlight important keywords for faster recall.