
Which one of the following is chloropicrin?
(A) 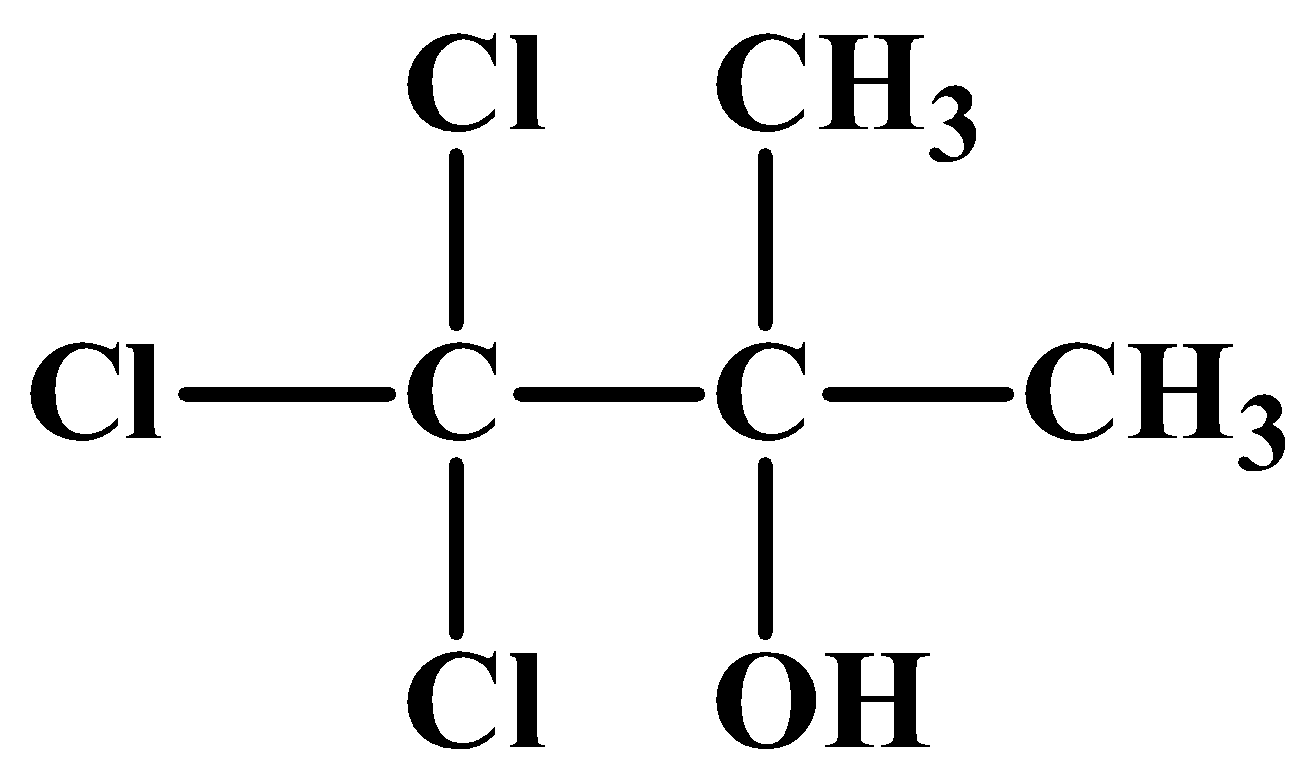
(B) 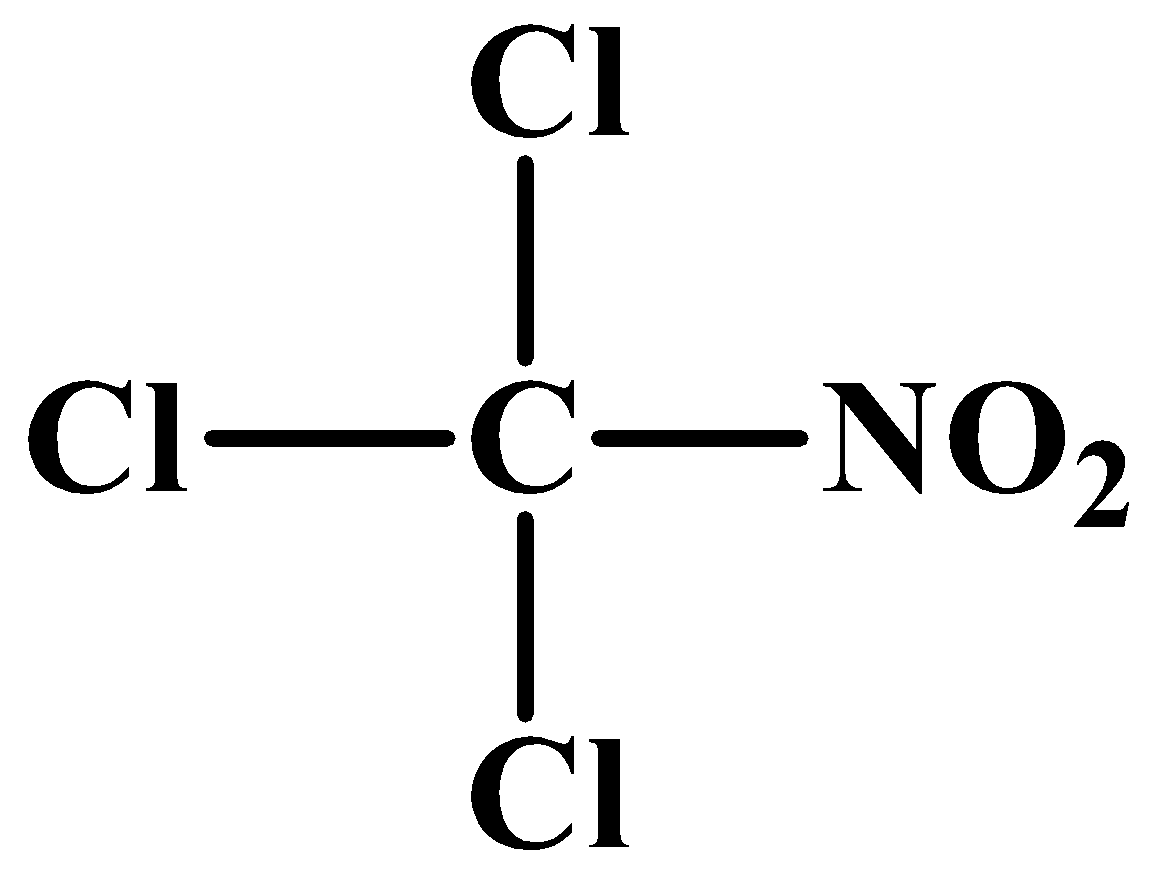
(C) 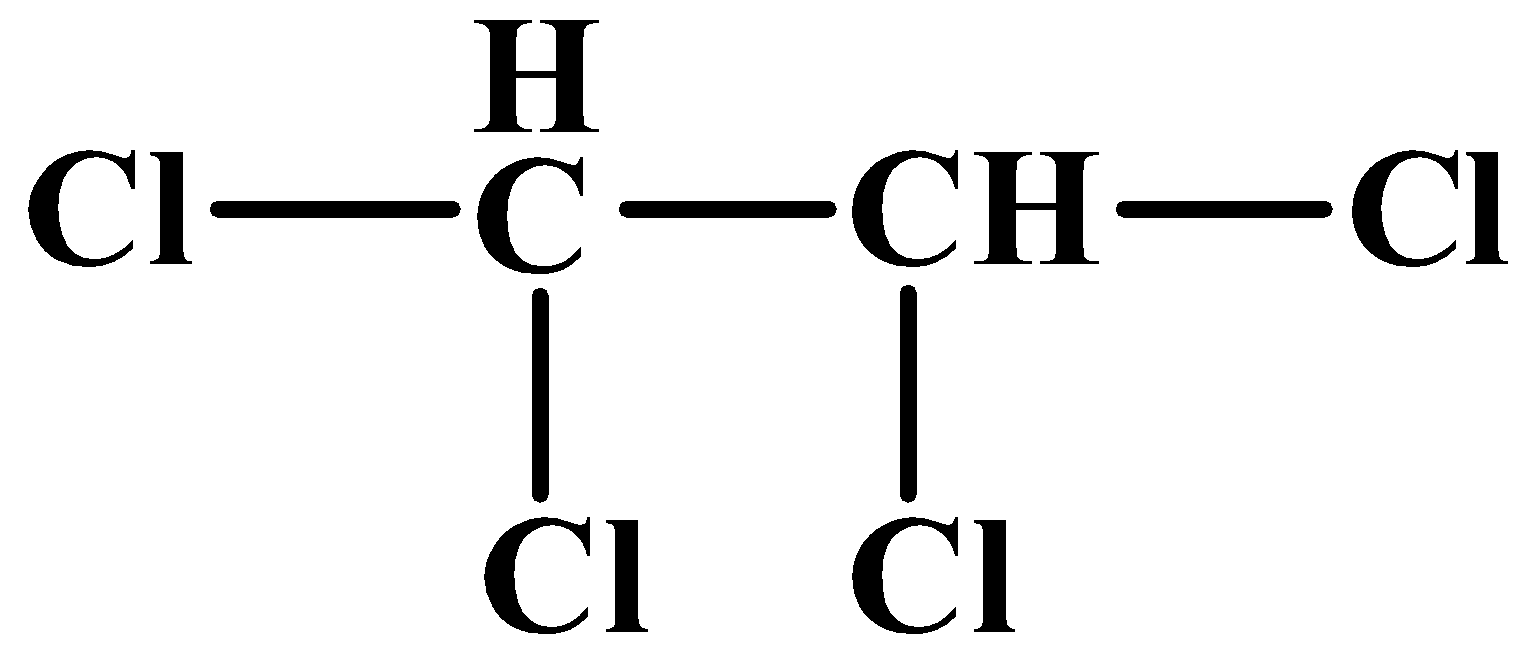
(D) 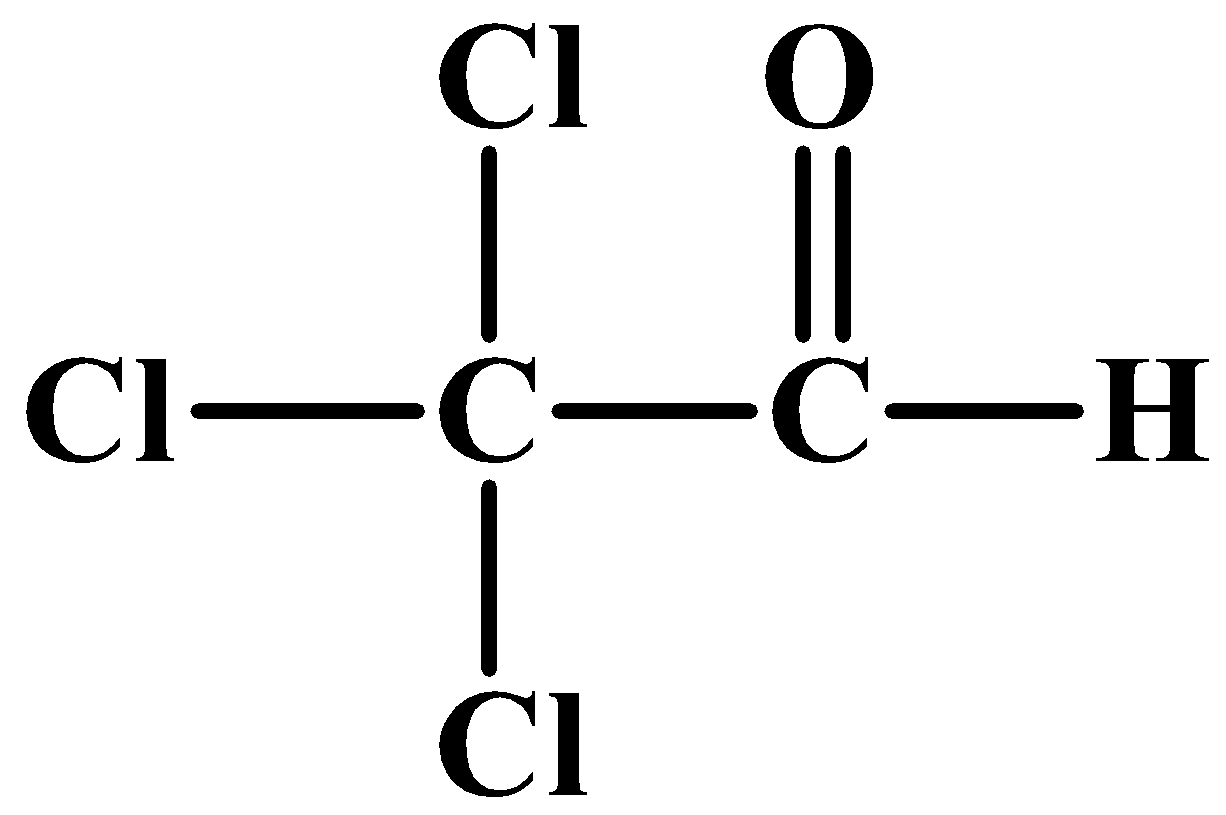
Answer
233.1k+ views
Hint: Try to recall that chloropicrin is one of the tear gas which is also called trichloronitromethane. It is a toxic and organic compound. Now, by using this you can easily find the correct option from the given ones.
Complete step by step solution:
It is known to you that there are different types of compounds that may be used as tear gas.
Tear gas is formally known as lachrymator agent or lachrymator (meaning “tear”).
When chloroform is heated strongly with concentrated nitric acid, then nitro chloroform is obtained. This is also called chloropicrin. The chemical formula of chloropicrin is\[CC{{l}_{3}}N{{O}_{2}}\].
The reaction for preparation of chloropicrin is as follows: \[CHC{{l}_{3}}+HN{{O}_{3}}\to CC{{l}_{3}}N{{O}_{2}}+{{H}_{2}}O\]
Chloropicrin also known as nitro chloroform is a chemical compound currently used as a broad-spectrum antimicrobial, fungicide, herbicide and insecticide.
It has the properties to irritate the mucosal membranes and causes draining of tears to remove the gas from eyes as soon as possible. It is also used as a tear war gas.
The vapours of chloropicrin are irritating to the skin, eyes and upper respiratory tract, and it has been used in chemical warfare.
Therefore, from above we can conclude that option B is the correct option.
Note: You should not forget that chloropicrin was used in world war as a chemical weapon and it was too dangerous for both animals and humans. It mainly affected the breathing mechanism.
It can also be prepared by the reaction of nitromethane with sodium hypochlorite.
Complete step by step solution:
It is known to you that there are different types of compounds that may be used as tear gas.
Tear gas is formally known as lachrymator agent or lachrymator (meaning “tear”).
When chloroform is heated strongly with concentrated nitric acid, then nitro chloroform is obtained. This is also called chloropicrin. The chemical formula of chloropicrin is\[CC{{l}_{3}}N{{O}_{2}}\].
The reaction for preparation of chloropicrin is as follows: \[CHC{{l}_{3}}+HN{{O}_{3}}\to CC{{l}_{3}}N{{O}_{2}}+{{H}_{2}}O\]
Chloropicrin also known as nitro chloroform is a chemical compound currently used as a broad-spectrum antimicrobial, fungicide, herbicide and insecticide.
It has the properties to irritate the mucosal membranes and causes draining of tears to remove the gas from eyes as soon as possible. It is also used as a tear war gas.
The vapours of chloropicrin are irritating to the skin, eyes and upper respiratory tract, and it has been used in chemical warfare.
Therefore, from above we can conclude that option B is the correct option.
Note: You should not forget that chloropicrin was used in world war as a chemical weapon and it was too dangerous for both animals and humans. It mainly affected the breathing mechanism.
It can also be prepared by the reaction of nitromethane with sodium hypochlorite.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




