
Which one of the following compounds does not react with bromine?
A. Ethylamine
B. Propene
C. Phenol
D. Chloroform
Answer
233.1k+ views
Hint: Bromination is a chemical reaction which involves the reaction of a compound with bromine where bromine gets added to the compound. The new compound formed after bromination shows new properties from that of the initial reactant.
Complete Step by Step Solution:
Ethylamine (or ethanamine) reacts with bromine \[{\rm{(B}}{{\rm{r}}_{\rm{2}}}{\rm{)}}\] in presence of aqueous sodium carbonate to form the products as N-bromoethanamine and hydrobromic acid.
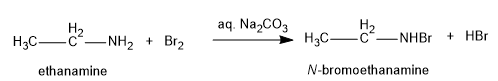
Image: reaction of ethylamine with bromine
Alkene is known to undergo bromination reaction through an addition reaction. Propene reacts with bromine \[{\rm{(B}}{{\rm{r}}_{\rm{2}}}{\rm{)}}\] to form the product as \[{\rm{1,2 - dibromopropane}}\]. Bromination in unsaturated compounds takes place via the formation of bromonium ion intermediate.
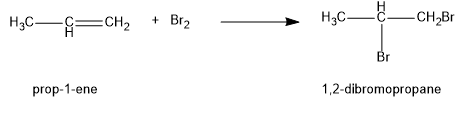
Image: reaction of propene with bromine
An aromatic compound undergoes bromination through electrophilic substitution mechanism. Phenol reacts with bromine \[{\rm{(B}}{{\rm{r}}_{\rm{2}}}{\rm{)}}\] to form the products as \[{\rm{2 - bromophenol}}\] and \[{\rm{4 - bromophenol}}\]. \[{\rm{4 - bromophenol}}\]is the major product due to less steric hindrance.
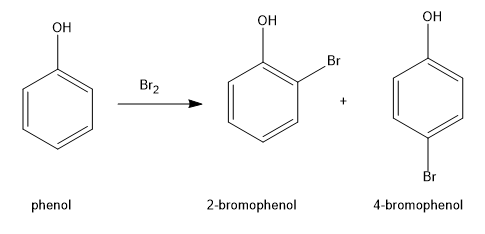
Image: reaction of phenol with bromine
Chloroform does not react with bromine \[{\rm{(B}}{{\rm{r}}_{\rm{2}}}{\rm{)}}\]since it has no \[\pi \]-unsaturation and hence cannot be nucleophilic. It might react with bromine unless energy in the form of heat or light \[(h\nu )\]is supplied.
\[{\rm{CHC}}{{\rm{l}}_{\rm{3}}}\,\, + \,\,{\rm{B}}{{\rm{r}}_{\rm{2}}} \to \,\,{\rm{No}}\,\,{\rm{reaction}}\]
Hence, chloroform \[{\rm{(CHC}}{{\rm{l}}_{\rm{3}}})\] does not react with bromine \[{\rm{(B}}{{\rm{r}}_{\rm{2}}}{\rm{)}}\].
Therefore, option D is correct.
Note: Depending on the type of reactant, bromination can occur in different ways. For example, a saturated compound undergoes bromination reaction through free radical mechanism, an unsaturated compound undergoes bromination through an addition reaction and an aromatic compound undergoes bromination process through electrophilic addition mechanism. Bromination has its use in chemical industries as a building block for various syntheses. Bromination may be used in pharmaceuticals, agricultural and chemical intermediates.
Complete Step by Step Solution:
Ethylamine (or ethanamine) reacts with bromine \[{\rm{(B}}{{\rm{r}}_{\rm{2}}}{\rm{)}}\] in presence of aqueous sodium carbonate to form the products as N-bromoethanamine and hydrobromic acid.

Image: reaction of ethylamine with bromine
Alkene is known to undergo bromination reaction through an addition reaction. Propene reacts with bromine \[{\rm{(B}}{{\rm{r}}_{\rm{2}}}{\rm{)}}\] to form the product as \[{\rm{1,2 - dibromopropane}}\]. Bromination in unsaturated compounds takes place via the formation of bromonium ion intermediate.

Image: reaction of propene with bromine
An aromatic compound undergoes bromination through electrophilic substitution mechanism. Phenol reacts with bromine \[{\rm{(B}}{{\rm{r}}_{\rm{2}}}{\rm{)}}\] to form the products as \[{\rm{2 - bromophenol}}\] and \[{\rm{4 - bromophenol}}\]. \[{\rm{4 - bromophenol}}\]is the major product due to less steric hindrance.

Image: reaction of phenol with bromine
Chloroform does not react with bromine \[{\rm{(B}}{{\rm{r}}_{\rm{2}}}{\rm{)}}\]since it has no \[\pi \]-unsaturation and hence cannot be nucleophilic. It might react with bromine unless energy in the form of heat or light \[(h\nu )\]is supplied.
\[{\rm{CHC}}{{\rm{l}}_{\rm{3}}}\,\, + \,\,{\rm{B}}{{\rm{r}}_{\rm{2}}} \to \,\,{\rm{No}}\,\,{\rm{reaction}}\]
Hence, chloroform \[{\rm{(CHC}}{{\rm{l}}_{\rm{3}}})\] does not react with bromine \[{\rm{(B}}{{\rm{r}}_{\rm{2}}}{\rm{)}}\].
Therefore, option D is correct.
Note: Depending on the type of reactant, bromination can occur in different ways. For example, a saturated compound undergoes bromination reaction through free radical mechanism, an unsaturated compound undergoes bromination through an addition reaction and an aromatic compound undergoes bromination process through electrophilic addition mechanism. Bromination has its use in chemical industries as a building block for various syntheses. Bromination may be used in pharmaceuticals, agricultural and chemical intermediates.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




