
Which of the following will not undergo H V Z reaction?
a. Propanoic acid
b. Ethanoic acid
c. 2-methyl propanoic acid
d. 2,2-dimethyl propanoic acid
Answer
522k+ views
Hint: Hell- Volhard- Zelinsky reaction is a type of odd substitution reaction in which carboxylic acid which contains $\alpha $hydrogen gets converted to $\alpha $ halo carboxylic acid.
Complete step by step answer:
First of all, let us understand what the Hell- Volhard- Zelinsky [H V Z] reaction?
Hell- Volhard- Zelinsky [H V Z] reaction is also known as H V Z reaction. It is a type of substitution reaction in which carboxylic acids are converted to $\alpha $halo carboxylic acid. The reaction is initiated by the addition of phosphorus tribromide [Catalytic amount] and the further addition of one molar equivalent of di-atomic bromine.
Since, it is clear that $\alpha $hydrogen present in carboxylic acid is substituted by a halo group so the presence of $\alpha $hydrogen in the corresponding carboxylic acid is very important.
Let us draw the structure of four carboxylic acids given to us in the question.
(a) Propanoic acid
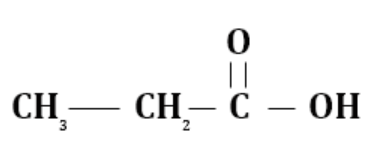
$(2 \propto \, - H)$
(b) Ethanoic acid
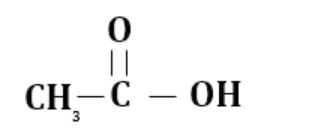
$(3 \propto \, - H)$
(c) 2-methyl propanoic acid
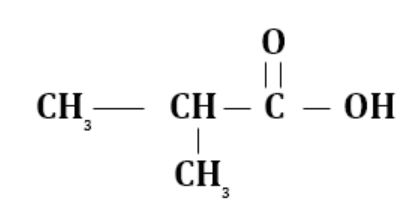
$(1 \propto \,H)$
(d) 2,2-dimethyl propanoic acid

$(no \propto \, - H)$
Thus, 2,2-dimethyl propanoic acid does not contain any $\alpha $hydrogen. Hence it will not participate in Hell- Volhard- Zelinsky [H V Z] reaction.
Option (d) is the correct answer.
Note:
Students should note that the H V Z reaction generally accomplishes bromination but fails in case of fluorination and iodination of carboxylic acid. The following reaction is not conducted at extremely high temperature, as there may be an elimination of hydrogen halide from the product thereby resulting in the formation of beta unsaturated carboxylic acid.
Complete step by step answer:
First of all, let us understand what the Hell- Volhard- Zelinsky [H V Z] reaction?
Hell- Volhard- Zelinsky [H V Z] reaction is also known as H V Z reaction. It is a type of substitution reaction in which carboxylic acids are converted to $\alpha $halo carboxylic acid. The reaction is initiated by the addition of phosphorus tribromide [Catalytic amount] and the further addition of one molar equivalent of di-atomic bromine.
Since, it is clear that $\alpha $hydrogen present in carboxylic acid is substituted by a halo group so the presence of $\alpha $hydrogen in the corresponding carboxylic acid is very important.
Let us draw the structure of four carboxylic acids given to us in the question.
(a) Propanoic acid
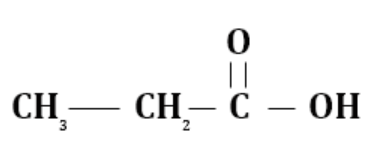
$(2 \propto \, - H)$
(b) Ethanoic acid
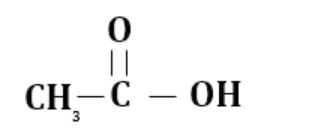
$(3 \propto \, - H)$
(c) 2-methyl propanoic acid

$(1 \propto \,H)$
(d) 2,2-dimethyl propanoic acid

$(no \propto \, - H)$
Thus, 2,2-dimethyl propanoic acid does not contain any $\alpha $hydrogen. Hence it will not participate in Hell- Volhard- Zelinsky [H V Z] reaction.
Option (d) is the correct answer.
Note:
Students should note that the H V Z reaction generally accomplishes bromination but fails in case of fluorination and iodination of carboxylic acid. The following reaction is not conducted at extremely high temperature, as there may be an elimination of hydrogen halide from the product thereby resulting in the formation of beta unsaturated carboxylic acid.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




