
Which of the following statements is not correct?
(1) \[{\left[ {CuC{l_4}} \right]^{2 - }}\] has a tetrahedral geometry and paramagnetic
(2) \[{\left[ {MnC{l_4}} \right]^{2 - }}\] has a tetrahedral geometry and is paramagnetic
(3) \[{\left[ {NiC{l_4}} \right]^{2 - }}\] has a tetrahedral geometry and is paramagnetic
(4) \[{\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}\] has a square planar geometry and is diamagnetic
Answer
233.1k+ views
Hint: We will apply the concept of Crystal Field Theory, which claims that the presence of Ligands causes the breakdown of orbital degeneracy in transition metal complexes. We will explain the geometry of given complexes using hybridization, as well as determine the nature of the ligand-based on its location in the spectrochemical series, i.e., whether it is a strong or weak ligand.
Complete Step by Step Solution:
As we know the atomic number of \[Cu\] is \[29\]so the electronic configuration will be:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^9}4{s^2}\]
But here copper exists in \[C{u^{2 + }}\] oxidation state so two electrons will be eliminated from \[4s\].
The electronic configuration will be:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^9}4{s^0}\]
To determine the hybridization, we draw the orbital as follows:
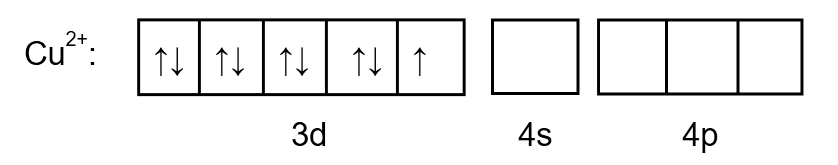
Image: \[C{u^{2 + }}\] ion electronic arrangement
Chlorine is a weak ligand so the pairing of electrons does not take place. Therefore, the electronic arrangement of \[{\left[ {CuC{l_4}} \right]^{2 - }}\]is as follows:
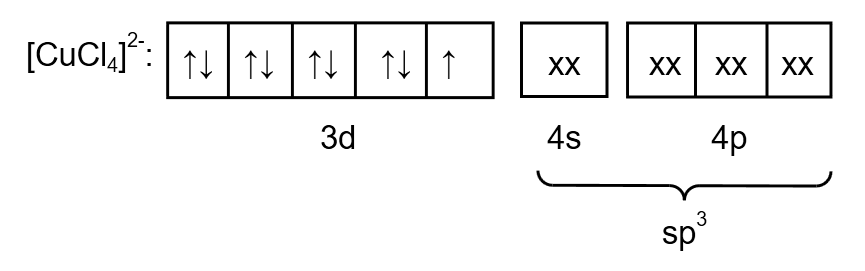
Image: electronic arrangement of \[{\left[ {CuC{l_4}} \right]^{2 - }}\]
From the electronic arrangement, the hybridization is \[s{p^3}\]. Therefore, the geometry is tetrahedral. As a compound contain one unpaired electron so the magnetic nature is paramagnetic.
Therefore, statement (1) is correct.
As we know the atomic number of \[Mn\] is \[25\] so the electronic configuration will be:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^5}4{s^2}\]
But here manganese exists in \[M{n^{2 + }}\] oxidation state so two electrons will be eliminated from \[4s\] .
The electronic configuration will be:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^5}4{s^0}\]
To determine the hybridization, we draw the orbital as follows:
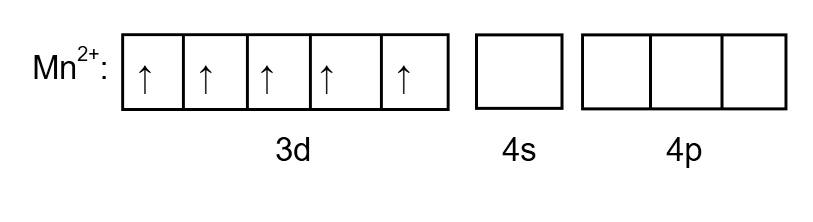
Image: \[M{n^{2 + }}\] electron arrangement
Chlorine is a weak ligand so the pairing of electrons does not take place. Therefore, the electronic configuration of \[{\left[ {MnC{l_4}} \right]^{2 - }}\] is as follows:
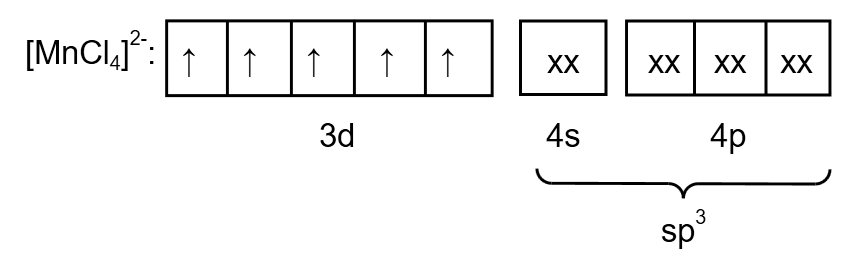
Image: electronic arrangement of \[{\left[ {MnC{l_4}} \right]^{2 - }}\]
From the electronic arrangement, the hybridization is \[s{p^3}\]. Therefore, the geometry is tetrahedral. As a compound contain one unpaired electron so the magnetic nature of \[{\left[ {MnC{l_4}} \right]^{2 - }}\] is paramagnetic.
Therefore, statement (2) is correct.
As we know the atomic number of \[Ni\] is \[28\] so the electronic configuration will be:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}4{s^2}\]
But here nickel exists in \[N{i^{2 + }}\] oxidation state so two electrons will be eliminated from \[4s\].
The electronic configuration will be:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}4{s^0}\]
To determine the hybridization, we draw the orbital as follows:
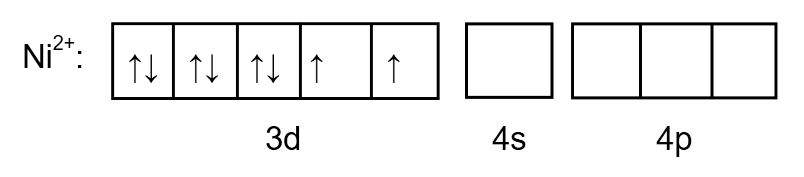
Image: \[N{i^{2 + }}\] electron arrangement
Chlorine is a weak ligand so the pairing of electrons does not take place. Therefore, the electronic configuration of \[{\left[ {NiC{l_4}} \right]^{2 - }}\] is as follows:
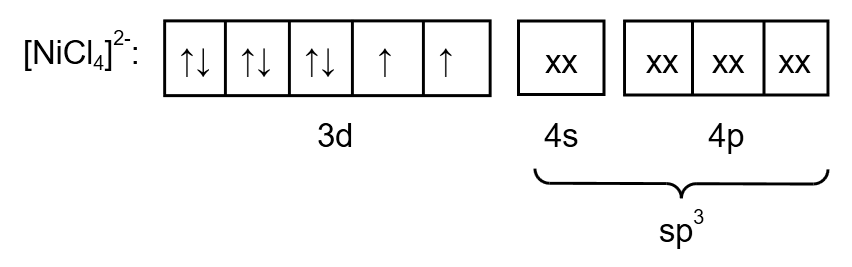
Image: electronic arrangement of \[{\left[ {NiC{l_4}} \right]^{2 - }}\]
From the electronic arrangement, the hybridization is \[s{p^3}\]. Therefore, the geometry is tetrahedral. As a compound contain one unpaired electron so the magnetic nature of \[{\left[ {NiC{l_4}} \right]^{2 - }}\] is paramagnetic.
Therefore, statement (3) is correct.
As we know the atomic number of \[Cu\] is \[29\]so the electronic configuration will be:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^9}4{s^2}\]
But here copper exists in \[C{u^{2 + }}\] oxidation state so two electrons will be eliminated from \[4s\].
The electronic configuration will be:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^9}4{s^0}\]
To determine the hybridization, we draw the orbital as follows:
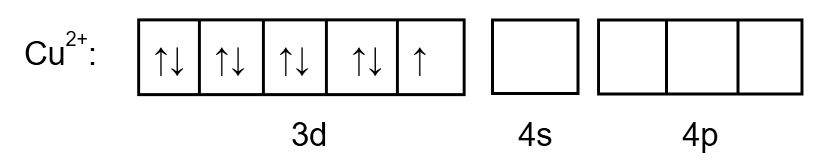
Image: \[C{u^{2 + }}\] electronic arrangement
Ammonia is weak ligand so pairing of electrons does not take place. But here an electron is transferred from \[3d\] orbital to \[4p\] orbital. Therefore, the electronic arrangement of \[{\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}\]is as follows:
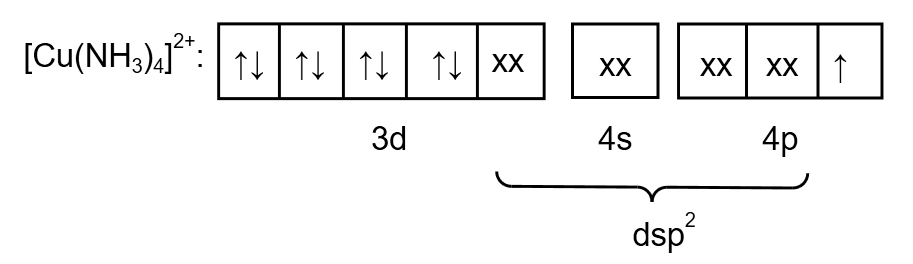
Image: electronic arrangement of \[{\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}\]
From the electronic arrangement, the hybridization is \[ds{p^2}\]. Therefore, the geometry is square planar. As a compound contains one unpaired electron so the magnetic nature of \[{\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}\] is paramagnetic.
Therefore, statement (4) is incorrect.
Note: With tetrahedral geometry, \[{\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}\] should form \[s{p^3}\] hybridization, however, this does not happen since the \[3d\] orbital only has one unpaired electron, therefore the unpaired electron is transferred to the \[4p\] orbital for increased stability. The Valence Bond Theory could not explain this cause of transference or the supply of energy required for electron transfer.
Complete Step by Step Solution:
As we know the atomic number of \[Cu\] is \[29\]so the electronic configuration will be:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^9}4{s^2}\]
But here copper exists in \[C{u^{2 + }}\] oxidation state so two electrons will be eliminated from \[4s\].
The electronic configuration will be:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^9}4{s^0}\]
To determine the hybridization, we draw the orbital as follows:

Image: \[C{u^{2 + }}\] ion electronic arrangement
Chlorine is a weak ligand so the pairing of electrons does not take place. Therefore, the electronic arrangement of \[{\left[ {CuC{l_4}} \right]^{2 - }}\]is as follows:

Image: electronic arrangement of \[{\left[ {CuC{l_4}} \right]^{2 - }}\]
From the electronic arrangement, the hybridization is \[s{p^3}\]. Therefore, the geometry is tetrahedral. As a compound contain one unpaired electron so the magnetic nature is paramagnetic.
Therefore, statement (1) is correct.
As we know the atomic number of \[Mn\] is \[25\] so the electronic configuration will be:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^5}4{s^2}\]
But here manganese exists in \[M{n^{2 + }}\] oxidation state so two electrons will be eliminated from \[4s\] .
The electronic configuration will be:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^5}4{s^0}\]
To determine the hybridization, we draw the orbital as follows:

Image: \[M{n^{2 + }}\] electron arrangement
Chlorine is a weak ligand so the pairing of electrons does not take place. Therefore, the electronic configuration of \[{\left[ {MnC{l_4}} \right]^{2 - }}\] is as follows:

Image: electronic arrangement of \[{\left[ {MnC{l_4}} \right]^{2 - }}\]
From the electronic arrangement, the hybridization is \[s{p^3}\]. Therefore, the geometry is tetrahedral. As a compound contain one unpaired electron so the magnetic nature of \[{\left[ {MnC{l_4}} \right]^{2 - }}\] is paramagnetic.
Therefore, statement (2) is correct.
As we know the atomic number of \[Ni\] is \[28\] so the electronic configuration will be:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}4{s^2}\]
But here nickel exists in \[N{i^{2 + }}\] oxidation state so two electrons will be eliminated from \[4s\].
The electronic configuration will be:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}4{s^0}\]
To determine the hybridization, we draw the orbital as follows:

Image: \[N{i^{2 + }}\] electron arrangement
Chlorine is a weak ligand so the pairing of electrons does not take place. Therefore, the electronic configuration of \[{\left[ {NiC{l_4}} \right]^{2 - }}\] is as follows:

Image: electronic arrangement of \[{\left[ {NiC{l_4}} \right]^{2 - }}\]
From the electronic arrangement, the hybridization is \[s{p^3}\]. Therefore, the geometry is tetrahedral. As a compound contain one unpaired electron so the magnetic nature of \[{\left[ {NiC{l_4}} \right]^{2 - }}\] is paramagnetic.
Therefore, statement (3) is correct.
As we know the atomic number of \[Cu\] is \[29\]so the electronic configuration will be:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^9}4{s^2}\]
But here copper exists in \[C{u^{2 + }}\] oxidation state so two electrons will be eliminated from \[4s\].
The electronic configuration will be:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^9}4{s^0}\]
To determine the hybridization, we draw the orbital as follows:

Image: \[C{u^{2 + }}\] electronic arrangement
Ammonia is weak ligand so pairing of electrons does not take place. But here an electron is transferred from \[3d\] orbital to \[4p\] orbital. Therefore, the electronic arrangement of \[{\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}\]is as follows:

Image: electronic arrangement of \[{\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}\]
From the electronic arrangement, the hybridization is \[ds{p^2}\]. Therefore, the geometry is square planar. As a compound contains one unpaired electron so the magnetic nature of \[{\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}\] is paramagnetic.
Therefore, statement (4) is incorrect.
Note: With tetrahedral geometry, \[{\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}\] should form \[s{p^3}\] hybridization, however, this does not happen since the \[3d\] orbital only has one unpaired electron, therefore the unpaired electron is transferred to the \[4p\] orbital for increased stability. The Valence Bond Theory could not explain this cause of transference or the supply of energy required for electron transfer.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




