
Which of the following statements is false
(A) $CaOC{{l}_{2}}$ gives $OH^-$,$Cl^-$ and $OCl^-$ in aqueous solution
(B) Allotropes of carbon include diamond and graphite.
(C) Bleaching action of $C{{l}_{2}}$in moist conditions is not permanent
(D) Calomel is $H{{g}_{2}}C{{l}_{2}}$
Answer
233.1k+ views
Hint: There are four statements in the question. We must determine which is false. For this, we have to check the correctness of each of the statements.
Complete Step by Step Answer:
(A) $CaOC{{l}_{2}}$ gives $OH^-$,$Cl^-$ and $OCl^-$ in aqueous solution.
$CaOC{{l}_{2}}$ is the formula for bleaching powder. It is also known as calcium oxychloride. $CaOC{{l}_{2}}$ in aqueous solution can be written as $Ca{{(OCl)}_{2}}.Ca{{(OH)}_{2}}.CaC{{l}_{2}}.2{{H}_{2}}O$which will give $OH^-$,$Cl^-$ and $OCl^-$ . The given statement above is true. As a result, it is incorrect.
(B) Allotropes of carbon include diamond and graphite.
Pure carbon has three allotropes – diamond, graphite, and fullerene. Graphite is a soft, black, and slippery solid and is mostly used in lubricants or pencils. Diamond is very hard and is a good thermal conductor. Diamond dust is used nowadays in beauty products like facials as it has the property to reduce wrinkles. It is highly expensive. However, the purest allotrope of carbon is fullerene. The given statement above is true. As a result, it is incorrect.
(C) Bleaching action of $C{{l}_{2}}$ in moist conditions is not permanent.
This statement is false as the bleaching action of $C{{l}_{2}}$in moist conditions is permanent.
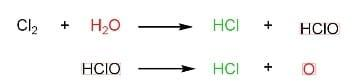
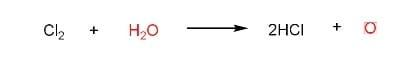
Coloured matter in the presence of nascent oxygen gives colourless matter.
(D) Calomel is $H{{g}_{2}}C{{l}_{2}}$
This statement is true. The calomel is mainly used as a saturated calomel electrode to measure potentials or pH in solutions.
Correct Option: (C) Bleaching action of $C{{l}_{2}}$in moist conditions is not permanent.
Note: All the other three options except (C) are true. Only the option (C) is false. Hence, it is the correct option.
Complete Step by Step Answer:
(A) $CaOC{{l}_{2}}$ gives $OH^-$,$Cl^-$ and $OCl^-$ in aqueous solution.
$CaOC{{l}_{2}}$ is the formula for bleaching powder. It is also known as calcium oxychloride. $CaOC{{l}_{2}}$ in aqueous solution can be written as $Ca{{(OCl)}_{2}}.Ca{{(OH)}_{2}}.CaC{{l}_{2}}.2{{H}_{2}}O$which will give $OH^-$,$Cl^-$ and $OCl^-$ . The given statement above is true. As a result, it is incorrect.
(B) Allotropes of carbon include diamond and graphite.
Pure carbon has three allotropes – diamond, graphite, and fullerene. Graphite is a soft, black, and slippery solid and is mostly used in lubricants or pencils. Diamond is very hard and is a good thermal conductor. Diamond dust is used nowadays in beauty products like facials as it has the property to reduce wrinkles. It is highly expensive. However, the purest allotrope of carbon is fullerene. The given statement above is true. As a result, it is incorrect.
(C) Bleaching action of $C{{l}_{2}}$ in moist conditions is not permanent.
This statement is false as the bleaching action of $C{{l}_{2}}$in moist conditions is permanent.


Coloured matter in the presence of nascent oxygen gives colourless matter.
(D) Calomel is $H{{g}_{2}}C{{l}_{2}}$
This statement is true. The calomel is mainly used as a saturated calomel electrode to measure potentials or pH in solutions.
Correct Option: (C) Bleaching action of $C{{l}_{2}}$in moist conditions is not permanent.
Note: All the other three options except (C) are true. Only the option (C) is false. Hence, it is the correct option.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




