
Which of the following sets of reactants is used for the preparation of paracetamol from phenol?
(A) $HN{{O}_{3}},{{H}_{2}}/Pd,{{(C{{H}_{3}}CO)}_{2}}O$
(B) ${{H}_{2}}S{{O}_{4}},{{H}_{2}},Pd,{{(C{{H}_{3}}CO)}_{2}}O$
(C) ${{C}_{6}}{{H}_{5}}{{N}_{2}}Cl,SnC{{l}_{2}},{{(C{{H}_{3}}CO)}_{2}}O$
(D) $B{{r}_{2}}/{{H}_{2}}O,Zn/HCl,{{(C{{H}_{3}}CO)}_{2}}O$
Answer
233.1k+ views
Hint: Phenol undergoes nitration to form p-nitrophenol. p-nitrophenol undergoes reduction then acetylation to form paracetamol, Nitric acid is used to add nitro groups on phenol. Acetic anhydride adds an acetylation group.
Complete step by step solution:
In paracetamol, para indicates acetyl group is present at para position with respect to a hydroxyl group and ol at the end indicates hydroxyl group.
The IUPAC name of Paracetamol is N-(4-hydroxyphenyl)ethanamide.
Sulphuric acid is used in sulphonation reactions. Zinc and hydrochloric acid, tin chloride and Hydrogen palladium are reducing agents and are used in reduction reactions.
Acetic anhydride is used as acetylating agent, it replaces a hydrogen atom with an acetyl group.
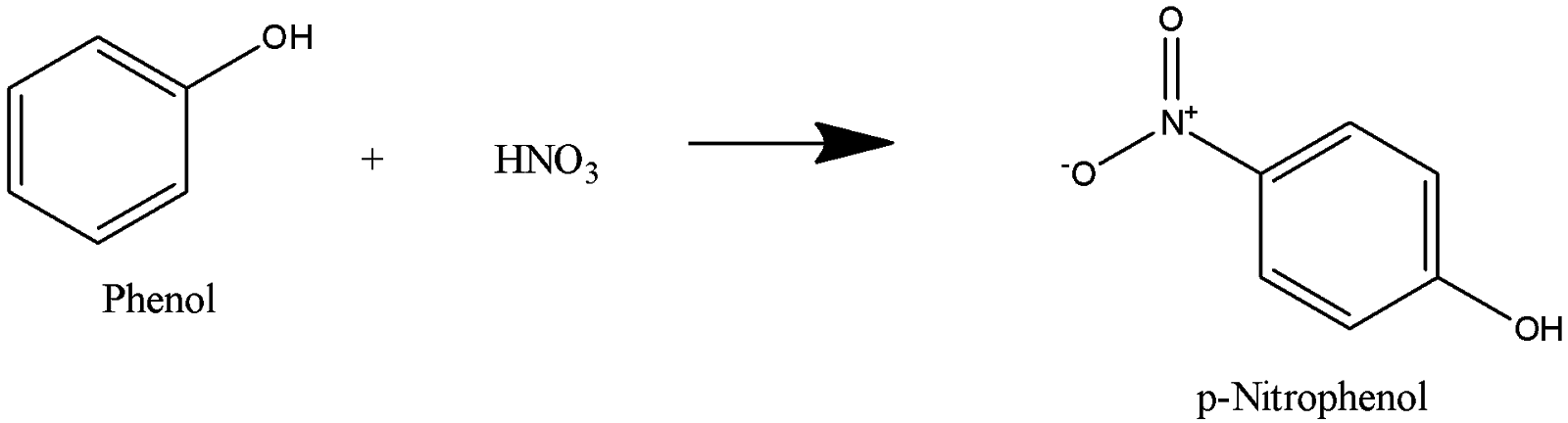
Hydroxyl group in phenol is ortho and para directing. When nitric acid is added to phenol, phenol undergoes nitration to form o-Nitrophenol and p-Nitrophenol, p-Nitrophenol is a major product.
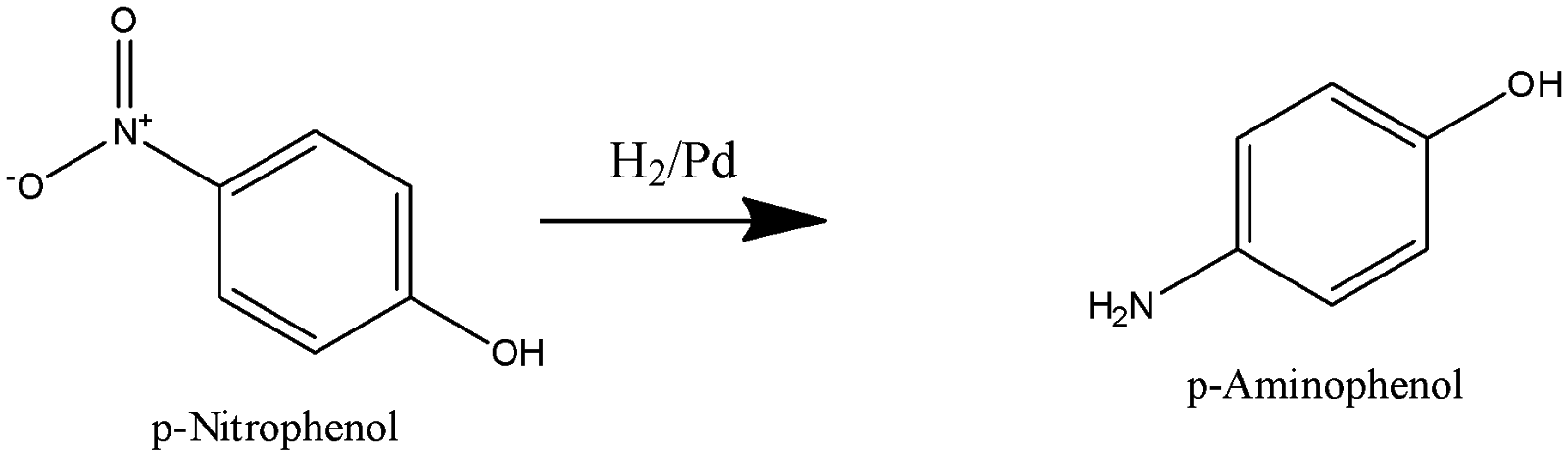
P-nitrophenol undergoes reduction to form 4-Aminophenol. Hydrogen is added in presence of palladium, which acts as a reducing agent and nitro group is reduced to Amino group.
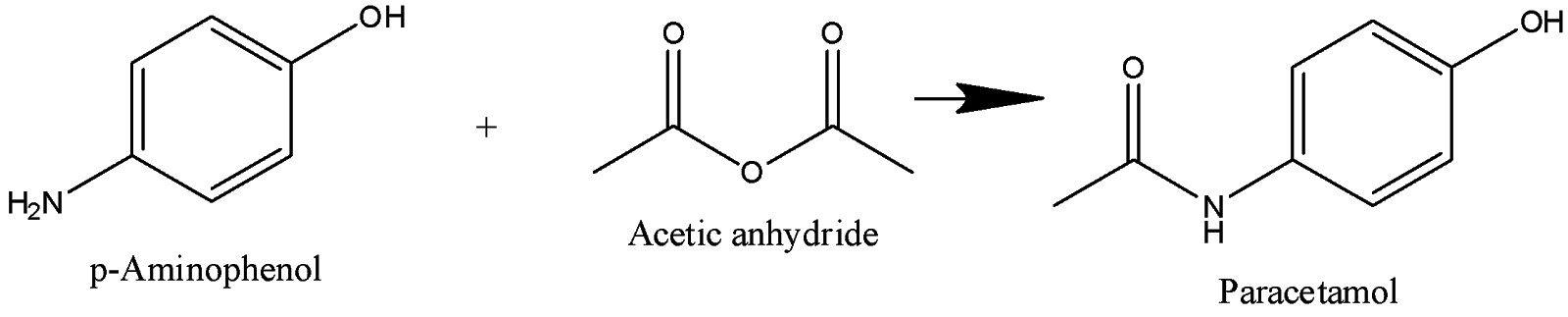
p-Aminophenol undergoes acetylation to form paracetamol. Acetic anhydride is an acetylating agent, Hydrogen atom of an amino group is replaced by acetyl group and paracetamol is produced.
Hence, option (A) is the correct anser.
Note: Hydroxyl group in phenol is ortho and para directing. p-Nitrophenol is a major product. and the Nitro group is reduced to an Amino group. The amino group undergoes acetylation to form paracetamol. Nitric acid is used in nitration of phenol.
Complete step by step solution:
In paracetamol, para indicates acetyl group is present at para position with respect to a hydroxyl group and ol at the end indicates hydroxyl group.
The IUPAC name of Paracetamol is N-(4-hydroxyphenyl)ethanamide.
Sulphuric acid is used in sulphonation reactions. Zinc and hydrochloric acid, tin chloride and Hydrogen palladium are reducing agents and are used in reduction reactions.
Acetic anhydride is used as acetylating agent, it replaces a hydrogen atom with an acetyl group.
Hydroxyl group in phenol is ortho and para directing. When nitric acid is added to phenol, phenol undergoes nitration to form o-Nitrophenol and p-Nitrophenol, p-Nitrophenol is a major product.

P-nitrophenol undergoes reduction to form 4-Aminophenol. Hydrogen is added in presence of palladium, which acts as a reducing agent and nitro group is reduced to Amino group.

p-Aminophenol undergoes acetylation to form paracetamol. Acetic anhydride is an acetylating agent, Hydrogen atom of an amino group is replaced by acetyl group and paracetamol is produced.

Hence, option (A) is the correct anser.
Note: Hydroxyl group in phenol is ortho and para directing. p-Nitrophenol is a major product. and the Nitro group is reduced to an Amino group. The amino group undergoes acetylation to form paracetamol. Nitric acid is used in nitration of phenol.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




